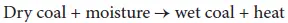The total exposed surface area of the coal is of importance in that the more area exposed, the better the chance of oxygen interacting or reacting uniting with the coal and any heat liberated in a given time for a given weight of coal will be higher (Elder et al ., 1945; Berkowitz and Speight, 1973).
When coal stockpiles are constructed by allowing mixed varied size coal to fall, roll, or slide, the larger pieces tend to collect at the bottom outside of the pile and the fines will collect at the top and inside of the stockpile. As a result, air will move easily through the outer parts of the stockpile but with much less freedom in the interior of the stockpile. Such a pile will allow the development of hot spots which can (or will) lead to spontaneous ignition of the coal with subsequent combustion of stockpile.
Moisture present in the coal is known to influence spontaneous heating in a stockpile insofar as the moisture affects ventilation (air flow) and pyrite reactivity. The higher the inherent (equilibrium) moisture content, the higher the heating tendency. The lower the ash free Btu, the higher the heating tendency. Coal with high oxygen content typically has a higher tendency to self-heat than coal of lower oxygen content. Thus, there appears to be an interaction between oxygen functions in the coal and aerial oxygen leading to a higher potential for formation of the coal-oxygen complex as the first stage of the self-heating process leading to a higher tendency for spontaneous ignition of the high-oxygen coal.
The effect of moisture on the self-ignition is twofold, thus (i) the vaporization of moisture consumes energy and hence the ignition process is impeded, (ii) promotion of self- ignition by the wetting of materials prone to evolution of heat during the moisture adsorption has been observed (Gray, 1990).
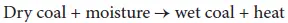
In addition to the heat of wetting, moisture simply blocks the access of oxygen through the coal pores. The water vapor diffusing outwards through the pores reduces the oxygen partial pressure and hence lowers the rate of the reaction or the polar water molecules attach to the reactive sites in coal (Jones, 1998).
The heat of condensation of coal in a stockpile can cause a rise in temperature in the pile, which is dependent upon the coal rank (Berkowitz and Schein, 1951). In addition, if dry screened coal is used as a storage-pile base for a shipment of wet coal, ignition can (or will) occur at the wet-dry interface of the two loads (Berkowitz and Speight, 1973). However, the more rapid oxidation occurring in high-moisture coals may be basically a function of coal rank rather than moisture content, since low-rank (high-oxygen) coal is usually also higher in moisture content.
Wetting and drying coal repeatedly may make it more susceptible to combustion. The actions of water may break up the coal, especially after freezing and thawing. Wet coal should not be piled or mixed with dry coal. Nor should coal be stored on a damp base. After heavy rains and snows (with accompanying snow melt) the stockpile should be inspected and observed for potential fires.
Thus, the moisture content of coal is also an important parameter in the rate of heat generation of the coal. Drying coal is an endothermic process, in which heat is absorbed, and the temperature of the coal is lowered. The adsorption of moisture on a dry coal surface is an exothermic process, with a heat-producing reaction. If coal is partially dried during its mining, storage, or processing, coal has the potential to re-adsorb moisture, thus producing heat. Therefore, the higher the moisture contents of the coal, the greater the potential for self-heating to occur. The most dangerous scenario for spontaneous combustion is when wet and dry coals are combined; the interface between wet and dry coal becomes a heat exchanger (Berkowitz and Schein, 1951; Smith et al ., 1991). If coal is either completely wet or completely dry, the risk is substantially reduced. In general, the moisture content of coal increases with decreasing rank.
The oxidation process commences once a fresh coal surface is exposed to air; however, the oxygen absorption rate is inversely proportional to time if the temperature remains constant. Therefore, if the coal is stockpiled so that the temperature in the pile does not rise appreciably insofar as the heat is removed at least as fast as it is generated by the oxidation process, the oxidation rate and, thus, the deterioration or weathering rate of the coal will lessen with time, but nevertheless, deterioration of coal properties during storage may be a major issue for the ultimate use of the coal (Porter and Ovitz, 1917; Vaughn and Nichols, 1985).
4.5 Mechanism of Spontaneous Ignition
Spontaneous combustion of coal is an important problem in its mining, long-distance transportation, and storage, in terms of both safety and economics. This is because coal reacts with oxygen in the air and an exothermic reaction occurs, even in ambient conditions. A problem arises when the rate of heat release produced by this process is more than dissipated by heat transfer to the surroundings. The heat of reaction accumulates, the reaction becomes progressively faster, and thermal runaway may take place to the point of ignition. It is for these reasons that the phenomenon of spontaneous combustion of coal has been of fundamental and practical importance to scientists.
There have been considerable difficulties in understanding the mechanism of the spontaneous ignition and spontaneous combustion of coal because of the involvement of many internal and external factors which affect the initiation and development of the phenomenon (Kröger and Beier, 1962; Güney, 1968; Beier, 1973; Chamberlain and Hall, 1973; Didari and Ökten, 1994; Kim, 1997; Kaymakçi and Didari, 2002).
However, large-scale and laboratory studies of the spontaneous ignition and combustion of coal have shown that high-volatile C bituminous coals exhibited high spontaneous combustion potentials in laboratory-scale tests. The results of these tests showed that the self-heating of a large coal mass depends not just on the reactivity of the coal, but also on the particle size of the coal, the freshness of the coal surfaces, the heat-of-wetting effect, and the availability of oxygen at optimum ventilation rates (Smith et al ., 1991; Kim, 1997).
In addition, several theoretical and experimental studies have been performed on coal spontaneous combustion (Van Doornum, 1954; Nordon, 1979; Schmal et al ., 1985; Brooks and Glasser, 1986; Arisoy and Akgun, 1994; Akgun and Arisoy, 1994; Krishnaswamy et al ., 1996; Monazam et al ., 1998; Arisoy and Akgun, 2000; Akgun and Essenhigh, 2001; Diaconu et al ., 2011). The main purposes of modeling studies has been to develop methods for determining the conditions at which the coal pile could undergo spontaneous combustion, to predict the safe storage time under those conditions, and to determine the influences of factors contributing to the spontaneous ignition. However commendable such studies are, it is always necessary that, in order to achieve dependable results, theoretical models can only be successfully used to investigate coal self-heating and self-ignition if the theoretical models are supported by experimental investigations and by field investigations (Arisoy et al ., 2006).
First and foremost, the oxidation of coal is a solid-gas reaction, which happens initially when air passes over the coal surface. Attempts to model this phenomenon have met with some success (Akgun and Essenhigh, 2001; Sensogut and Ozdeniz, 2005). However, there is often the failure to recognize that the phenomenon of self-ignition followed by combustion is site specific and is dependent upon several criteria such as (i) the coal type, (ii) the construction of the stockpile, and, last but not least, (iii) the atmospheric conditions. Indeed, there is no reason to conclude that the self-ignition of coal in a surface stockpile has the same initiation mechanism as self-ignition of coal in an underground coal mine.
Читать дальше