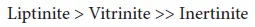In general, the critical temperature for bituminous coal in storage is approximately 50 to 66°C (122 to 150°F). From this temperature, heating will usually increase rapidly and may be unstable after which ignition occurs, unless preventive steps are taken. The basic chemical premise is that for every 10°C (18°F) the rate of a chemical reaction approximately doubles (for coal oxidation, the factor is 2.2). Hence oxidation leading to spontaneous ignition may appear to be (and often is) irreversible unless steps are taken to modify the oxidation reaction and the ensuing liberation of heat.
The petrographic composition of a coal is determined by the nature of the original plant material from which it was formed and the environment in which it was deposited rather than the degree of coalification (i.e., rank). The homogenous microscopic constituents of coal ( macerals , named by analogy of minerals in inorganic rocks) can be distinguished in three groups: (i) vitrinite consists of the remains of woody material, (ii) liptinite – formerly called exinite – consists of the remains of spores, resins and cuticles, and (iii) inertinite consists of the remains of oxidized plant material.
At constant rank, as the inertinite content of a coal increases, the self-heating propensity of the coal decreases. The general trend also indicates an increase in self-heating propensity with increasing vitrinite and/or liptinite content. Thus, the ease of oxidation of coal macerals is:
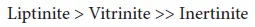
However, coal rank seems to play a more significant role in self-heating than the petrographic composition of coal (Speight, 2013).
Finally, spontaneous ignition and spontaneous combustion of coals also causes a serious problem for coal producers and users during transportation and storage ( Chapters 3, 4) (Nugroho et al ., 2000). Improvements to low-rank coal are made by either thermal drying or through blend with higher-rank coals. Thermal drying of moist lower-rank coals could increase the calorific value of a product whilst blending of coals of different types offers a greater flexibility and economic benefit. However, the problem of spontaneous ignition and combustion assumes even greater significance since the removal of moisture can enhance the potential for spontaneous ignition and combustion. The risk of spontaneous combustion is also made greater during blending and when storage of such lower-rank coals takes place. This is particularly the case with low-sulfur subbituminous coals which are now used to meet emission limits.
The primary source of heat generation within coal stockpiles is the exothermic low- temperature oxidation reaction, while mass and heat transport play a major role in determining the magnitude of the temperature rise in a given situation. Despite the extensive previous works on spontaneous ignition of coal using various techniques, the effect of particle size in the case of single-type coals on the rate of low-temperature oxidation, remains controversial (Nugroho et al ., 2000).
4.4.2 Pyrite and Other Minerals
Sulfur, once considered a major factor, is now thought to be a minor factor in the spontaneous heating of coal. There are many very low-sulfur western subbituminous coals and lignite that have high oxidizing characteristics and there are high-sulfur coals that exhibit relatively low oxidizing characteristics.
However, pyrite (FeS 2) evolves heat from aerial oxidation and was believed to be the cause of the spontaneous heating of coal. The heat generation locally promotes the self-heating process of coal but the reaction products have a greater volume than the original pyrite, with the result of breaking open any coal in which they are embedded and thus exposing a greater surface of coal to the air.
The interaction of pyrite (FeS 2) with water and oxygen is also an exothermic reaction and results in the formation of iron sulfate (FeSO 4) and sulfuric acid (H 2SO 4). Thus, if coal is stored in the open, rain will most likely increase the rate of this reaction and for the same reason water-flooding (to extinguish fires) may also increase the rate of the reaction.
Pyrite, through its transformation to bulkier materials, has also been cited as responsible in some cases for slacking and the resultant production of fines. For these reasons, coal users are generally reluctant to stockpile high-sulfur coal for extended periods of time (Berkowitz and Schein, 1951). However, stockpiling low-sulfur content of coal is no guarantee of safe storage – coal with low sulfur content can also spontaneously ignite.
Many minerals affect the oxidation rate to some extent, either accelerating or inhibiting it. Alkali chemicals are capable of accelerating the rate of the oxidation reaction while borates and calcium chloride can act as retardants of the reaction rate. The oxidation process is also promoted if ankerite [a calcium, iron, magnesium, manganese carbonate mineral of the group of rhombohedral-shaped carbonates, i.e., Ca(Fe.Mg.Mn)(CO 3) 2] is a constituent of the coal mineral matter. In contrast to ankerite, the presence of silica and alumina minerals tends to retard the oxidation reaction.
4.4.3 Coal Size and Stockpile Ventilation
Oxidation increases with increasing fineness (decreasing size) of the coal pieces and the rate of oxidation of coal with oxygen of air is proportional to the specific internal surface (or external surface area). For the internal surface, the proportional coefficient at low temperatures is the cube root but analysis also shows that both rate and extent of oxidation increase with the decrease in particle size, until a critical particle diameter is reached, below which the rate remains fairly constant. For the external surface area, the surface area of a ton of half-inch particles is greater that the surface area of a ton of one-inch particles of coal.
The natural ventilation in coal storage piles is generally adequate to remove sensible heat as fast as it is liberated in the oxidation process. However, in situations where the ventilation is adequate to maintain oxidation but inadequate to dissipate the heat produced, the coal absorbs the heat, causing a rise in the internal temperature of the stockpile. A chain reaction follows in which the oxidation rate increases with increasing temperature and, if the temperature rise is allowed to proceed unchecked in the stockpile, the ignition temperature of the coal will eventually be reached and the stockpile will begin to burn. To an external observer, it will at first appear that the coal is smoldering die to emission of light barely visible smoke but in reality, the fire inside the stockpile may be vicious and vigorous.
Run-of-mine coal (ROM coal) is difficult to store because of the large percentages of fines mixed with the lump, of which some may be minerals that promote the oxidation and, thus, spontaneous ignition. On the other hand, there is usually less danger in storing lump coals that have been double-screened or closely sized. The uniform pieces of coal are honeycombed with passages through which air can circulate freely and carry off the heat generated. However, in stockpiles of coal fines sufficiently compacted so as to exclude air, the potential for spontaneous ignition is diminished. But it must be recognized that stockpiles of coal (whatever the size of the coal) are subject to some degree of oxidation and, when the auspices are correct, spontaneous ignition.
If coal is to be stored for prolonged periods of time, the pile should be constructed so that air (in the case of fine coal or mixed sizes such as run-of-mine coal) is excluded. On the other hand, if the coal is to be stored as lump coal, air should be allowed to circulate freely through the pile.
Читать дальше