Run-of-mine coal generally has mineral matter on the order of 5 to 40% w/w and sulfur on the order of content of 0.2 to 0.8% w/w% depending on the geologic conditions and mining technique used. Coal cleaning, therefore, is often required to remove excessive impurities for efficient and environmentally safe utilization of coal. One important purpose of coal preparation is to increase the heating value of the coal by mechanical removal of impurities. This is often required in order to find a market for the product. Run-of-mine coal from a modern mine may incorporate as much as 60% reject materials. In the United States, the coal cleaning is most common at eastern and midwestern mines.
Current commercial coal cleaning methods are invariably based on physical separation; chemical and biological methods tend to be too expensive. Typically, density separation is used to clean coarse coal while surface property-based methods are usually preferred for fine coal cleaning (Davis, 1993; Dodson et al ., 1994). In the density-based processes, coal particles are added to a liquid medium and then subjected to gravity or centrifugal forces to separate the organic-rich (float) phase from the mineral-rich (sink) phase.
Density-based separation is commercially accomplished by the use of jigs, mineral spirals, concentrating tables, hydrocyclones, and heavy media separators. The performance of density-based cleaning circuits is estimated by using laboratory float-sink tests. In the surface property-based processes, ground coal is mixed with water and a small amount of collector reagent is added to increase the hydrophobicity of coal surfaces. Subsequently, air bubbles are introduced in the presence of a frothing agent to carry the coal particles to the top of the slurry, separating them from the hydrophilic mineral particles. Commercial surface property-based cleaning is accomplished through froth or column flotation.
Other physical coal cleaning methods include selective agglomeration, heavy and medium cycloning, and dry separation with electrical and magnetic methods (Couch, 1991, 1995). Selective agglomeration and advanced cycloning have the high probability of commercialization, particularly for reducing the sulfur content of coal (Couch 1995). In selective agglomeration, the coal is mixed with oil. The oil wets the surface of coal particles and thus causes them to stick together to form agglomerates. The agglomerated coal particles are then separated from the mineral particles that stay in suspension because they do not attract oil to their surfaces.
3.5.1 Effect of Composition and Rank
When coals are combusted or pyrolyzed, there is the near complete elimination of hydrogen and oxygen – the carbon residue that remains, however, still contains small quantities of nitrogen and sulfur. Typical values for carbon, hydrogen, volatile matter (i.e., low molecular weight products of the pyrolysis), and residual (fixed, non-volatile) carbon of the various classes of coal ( Table 3.2) provide the basis of a system for describing coal rank (Speight, 2013).
In the context of coal cleaning, oxygen is often considered unimportant because it is nonpolluting! But some consideration should be given to the effect of oxygen on the fate of its nitrogen and sulfur compatriots as well as its effect when water (a product of the combustion of hydrocarbons which also contain oxygen) condenses with other by-products on the cooler parts of combustion systems; corrosive, aqueous acids can be the result.
Sulfur is a special case because it is considered to be, and actually is, a more a serious pollutant than oxygen. Sulfur occurs in various forms and is distributed throughout the organic matrix and in the minerals. As organic sulfur, it occurs in the organic structure of the coal and as pyritic sulfur, it occurs as discrete particles of pyrite (Fe). In addition, sulfates are occasionally found in the minerals. In summary, the bulk of the sulfur (organic and/or inorganic) present in coal has the potential to occur as gaseous combustion products.
Rank has been assumed to have an effect on the extent of hydrophobic character of coal (Speight, 2013). However, recent work on the prediction of coal hydrophobicity indicates that this property correlates better with the moisture content than with the carbon content and better with the moisture/carbon molar ratio than with the hydrogen/ carbon or oxygen/carbon atomic ratios (Labuschagne et al ., 1988). Thus, it appears that there is a relationship between the hydrophobicity of coal and the moisture content. But there are differences in the behavior of coals and the differences are usually referred to as wettability, which can be quantified by measurement of the contact angle of the solid with water.
Methods employed for the removal of impurities from coal depend on the physical differences between the impurity and the coal; one such example is the difference in specific gravity.
Coal preparation plants generally use gravity process equipment to separate the refuse from the coal. Most of the extraneous impurities mined with coal are much heavier than the coal itself – coal has a specific gravity between 1.35 and 1.5, while the refuse rock has a specific gravity on the order of 2.1 to 2.3 – and separation can be effected by immersing the run-of-mine coal in a fluid having a specific gravity greater than that of the coal but less than that of the impurity ( heavy media process ). This allows the coal to float and the heavy waste material to sink and the two products are collected separately (Figure 3.2) (Couch, 1991).
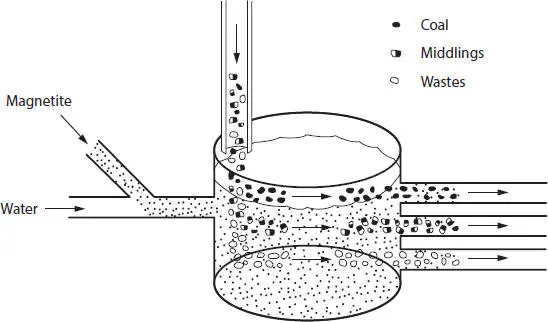
Figure 3.2A dense-medium separation method (Speight, 2013).
3.5.2.1 Dense Media Washing
The dense media washing process ( heavy media washing process ) is the most popular method of cleaning coarse sizes and jig plants are probably the second most common method used for coarse coal.
In a heavy-media washing plant, all the cleaning is done by flotation in a medium of selected specific gravity, maintained by a dispersion of finely ground magnetite in water. The incoming raw coal is separated at 1/4 inch on an inclined screen. The oversize material ( overs ) proceeds to a flat pre-wet screen, where the fine dust particles are sprayed off from the +1/4-inch coal. This increment is discharged into a heavy-medium vessel or bath, where the refuse is separated from the coal. The refuse is discharged to a “refuse rinse” screen, where it is dewatered. The use of magnetite has also been investigated in cyclone cleaning of coal (Klima et al ., 1990).
The freed medium is divided into two parts, one returning directly to circulation via the heavy-medium sump and the other pumped to magnetite recovery. The refuse is discharged from the screen for disposal. The coal is discharged from the washer to a coal-rinse screen, where the coal is dewatered and the medium is treated as from the refuse screen. The clean coal is then centrifuged, crushed, and loaded. The fine coal (less than 1/4 inch) from the raw coal screens is combined with magnetite and water and pumped to a heavy-media vessel in that the magnetite is finer and the effective specific gravity is different.
The refuse is dewatered and the medium is recovered, as in the coarse coal selection. The coal is discharged over a sieve bend and then proceeds to a centrifuge for final dewatering prior to transfer to a thermal dryer or to loading.
Читать дальше













