1.2.4.4 Chemical Absorption
The basic configuration of chemical absorption ( Figure 1.8) includes the reaction of a liquid solvent with CO 2in a column called absorber at a relatively low temperature, 40–60 °C, and its desorption in another column called desorber or stripper, generally at a high temperature, 100–140 °C. It must be noted that process modifications and solvent enhancements might modify those process conditions.
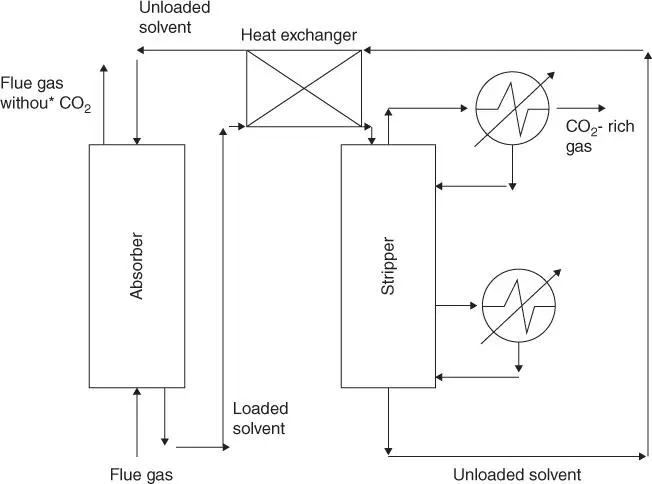
Figure 1.8General chemical absorption configuration
The absorption of CO 2into liquid solvents takes place by three phenomena: chemical reaction, physical absorption, and diffusivity. Depending on the compound and the conditions, one phenomenon will be predominant over the others.
Chemical solvents are more attractive candidates for typical post‐combustion processes, with relatively low partial pressures of CO 2(10–15% in coal power plants and 4–8% for gas‐fired power plants). Chemical absorption follows a standard configuration such as in Figure 1.8. However, new configurations have appeared to enhance the process, increase the efficiency, and/or decrease the capture costs.
Chemical absorption with amines is by far the most advanced carbon capture process and the only one that reached a TRL of 9 [2]. The most tested solvent is aqueous monoethanolamine (MEA) solution, although it does not represent any more the benchmark solution as consolidated alternatives show enhanced properties. Two large‐scale facilities have used enhanced systems, the Boundary Dam Capture plant [2] and Petra Nova. One of the main pathways to get more efficient chemical absorption processes and cut down costs is the development of new solvents. However, many solvents are emerging and only few have been tested at large scale, maintaining the TRL of other new systems still low. A review of commercial solutions and relevant projects can be found, for example, in Ref. [22]. The main criteria for the selection of a solvent are included in Table 1.2.
Primary amines are of high interest because of their fast reaction with CO 2. However, the main drawback is their high energy consumption for the solution regeneration. Several alternatives are emerging to decrease such penalty, the most common one being the use of tertiary amines. However, the CO 2absorption in tertiary amines is much slower. Consequently, other alternatives are emerging, such as the use of blends combining primary and tertiary amines (commonly called “promoted tertiary amines” or “activated tertiary amines”). Numerous alternatives have emerged during the past years; perhaps it is difficult to establish the best alternative.
Table 1.2Desired solvent properties and its impact on the absorption process [75].
Source: Adapted from Mathias et al. [75].
| Solvent property |
Impact on the absorption process |
| High capacity and low heat of absorption |
It is linked to the energy requirements per ton of CO 2, but the absorption capacity is connected to heat (thermodynamics) and independent variation is limited |
| High mass transfer and chemical kinetics |
It reduces equipment size or the capacity by operating near the equilibrium limit |
| Low viscosity |
It reduces the pumping costs and potentially increases the mass transfer and the heat transfer rate |
| Low degradation tendency |
It reduces the solvent make‐up and the regenerator can operate at higher pressure/temperature, increasing the thermal efficiency |
| Low toxicity/environmentally friendly |
It becomes more important if toxic by‐products are released during volatility losses |
| Cost and availability |
It will impact on reaching commercial scale |
| Low fouling tendency |
It will impact on the operation |
A potential substitute of traditional solvents is the use of compounds that, at unloaded or loaded conditions, separate into two phases, called biphasic solvents. There are two types of biphasic solvents, namely, liquid–liquid or solid–liquid, depending on the phases in solution. The main advantage is that only one phase needs to be regenerated, and consequently, the stripper size is reduced, and the energy consumption is potentially lower. Consequently, numerous biphasic solvents have been studied in the literature (e.g. in Ref. [23]).
Another strategy is to add enzymes, such as carbonic anhydrase (CA) [24]. CA increases the kinetic constant of the absorption of CO 2in aqueous amine and dilute carbonate solutions by catalyzing the CO 2hydration. The impact will depend on the compounds in solution, as the regeneration of the enzyme regeneration rate will vary. The challenges enzymes offer are their pH and thermal stability, lifetime, and sensitivity to pollutants such as SO xand NO x.
At lower development stage, solvents can be encapsulated in thin polymer shells and be considered as a bed of capsules containing the solvent. Capsules must be permeable enough to allow carbon dioxide to get in contact with the solvent but strong enough to resist the high regeneration temperatures during a number of cycles [25]. The benefit of this configuration is to increase the surface area of the solvent in contact with the flue gas and avoid issues related to viscosity and precipitation.
Recently, ionic liquids (ILs) are of great interest. These are composed of ions and are at liquid state below 100 °C. If the melting point is below the room temperature, these are referred as room temperature ionic liquids (RTILs). These solvents are recognized by their low vapor pressure, high thermal and chemical stability, nonflammability, and high viscosity. These properties open new possibilities for the solvent regeneration at different pressures and temperatures, which can be optimized accordingly. Some ILs show a high absorption capacity, although the viscosity could be decremental for the mass transfer.
Physical solvents are characterized for the high physical solubility of CO 2in these and are especially interesting for flue gas with high CO 2content [26]. There are commercial processes based on this principle, such as Rectisol®, Selexol®, Purisol®, Morphysorb®, and Fluor®, particularly effective at high concentrations of acid gas, high pressure, and low temperature [27] and are characterized by their low vapor pressure, low toxicity, and low corrosion [15].
An emerging pathway is the use of hybrid solvents, solutions containing amine/s and organic compound/s with or without the presence of water, the former called as water‐lean solvents. The goal is to maintain an enhanced physical absorption by substituting partial/totally the water content and maintaining a considerable chemical reaction by keeping the amine in the solution. It is known that at low concentration of the amine(s), the physical solubility plays an important role and the diffusivity can also become an important factor in viscous solutions. The enhanced solubility of CO 2in organic solvents, compared to water, has been widely studied in the past [28–31], and this presents advantages in its application in chemical absorption. During the desorption, the main energy penalty is due to the water evaporation. Decreasing the water content will decrease this energy penalty. Partial and total substitution of water by organic solvents has been considered as an alternative to decrease the steam consumption in the desorber. However, as studied in Ref. [32], the absorption kinetics would just be favorable, compared with aqueous amine solutions, at certain conditions of pressure and temperature in the absorber. The total substitution of water in water‐lean solvents will limit the reactions that take place in solution: hydrolysis will not occur and the carbamate and bicarbamate ions will be nonexistent [33]. However, the net benefit in the energy consumption when using water‐lean solvents is not yet clear, as discussed in Ref. [34].
Читать дальше













