1 ...6 7 8 10 11 12 ...31
1.2.4.4.1 Advances on Process Configurations
As mentioned previously, chemical absorption is the most advanced technology, reaching commercial status (TRL 9). However, there are still barriers that slow down its application in industrial and power sectors. Cost is one of the challenges to overcome and energy consumption has a strong contribution. The development of new solvents and improvements on the process flow sheet and/or its integration in the industrial or power facility could reduce this energy consumption.
The common process modifications can be divided as in Ref. [35]: (i) absorption enhancement, (ii) heat integration, and (iii) heat pumps. Perhaps these can also be classified by their purpose, as in Ref. [36]: (i) increase of rich solvent loading, (ii) reduction of the specific reboiler duty, or (iii) combination of both. The enhancement on the absorption and desorption processes and its impact on costs will depend on other factors such as the solvent and the facility. The modifications on the stripper to reduce energy consumption are being considered for the next generation of post‐combustion processes' configurations with advanced solvents (e.g. as in Ref. [37]).
1.2.5 Others CO 2Capture/Separation Technologies
Other CO 2capture/separation technologies such as electrochemical, cryogenic separation, liquefaction, microbial/microalgae, or direct air separation are described in the literature.
Hybrid technologies have been studied in the past years, aiming to achieve higher capture rates and/or sum up the advantages of each CO 2capture technology. The hybrid processes can be classified into absorption‐based, adsorption‐based, membrane‐based, and cryogen‐based hybrid processes. The integration of membranes into the absorption process (such as in the membrane contractor arrangement), catalysis process, and cryogenic process has progressed over the past years. However, the majority of the results are based on simulations or small‐scale testing campaigns, and the real value of using two technologies is not clear [38].
Within the range of emerging technologies, electrochemical separation has had a fast development over the past years and, potentially, will continue in this pathway. The following Section 1.2.5.1will be focused on fuel cells because of the growing expectation on this electrochemical separation technology for its integration in power plants.
Fuel cells convert chemical energy of a gaseous fuel directly into electricity and heat. The fuel is oxidized electrochemically, which leads to lower exergy losses compared to direct combustion. In general, fuel cells are classified by the electrolyte material and their operating temperature ( Figure 1.9). Low‐temperature fuel cells (100–250 °C) include alkaline fuel cells (AFCs), phosphoric acid fuel cells (PAFCs), and proton exchange membrane fuel cells (PEMFCs), while high‐temperature fuel cells (600–900 °C) refer to Molten carbonate fuel cells (MCFCs) and solid oxide fuel cells (SOFCs). Because of the high temperature at which MCFCs and SOFCs operate, natural gas reformation and the subsequent shift reaction can be performed in the fuel cell itself. MCFCs and SOFCs are most appropriate for stationary power production at scales ranging from a few hundred kilowatts up to a few megawatts because of their high electrical efficiencies and the ability for cogeneration of electricity and heat [39]. Moreover, SOFCs and MCFCs are more fuel flexible and are not poisoned by carbon monoxide and carbon dioxide.
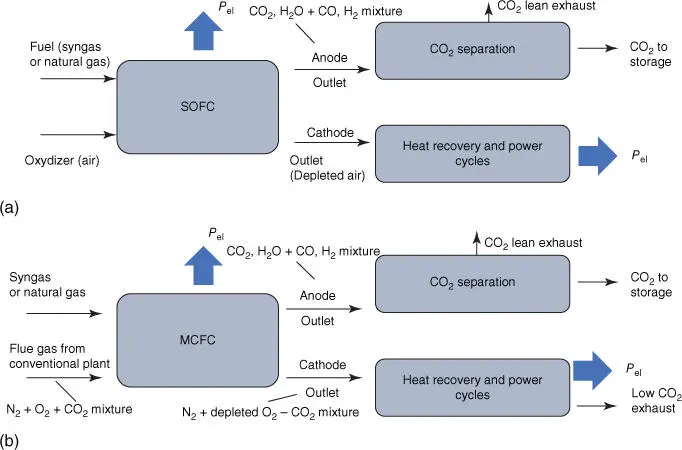
Figure 1.9Two main options for CO 2capture using fuel cells. (a) The FC oxidizes a fuel taking oxygen from air and later separating CO 2from the anode effluent. (b) The MCFC concentrates the CO 2in flue gas from a conventional power plant from the cathode inlet to the anode outlet, while also oxidizing a portion of additional fuel.
Source: Adapted from [11].
When MCFCs/SOFCs are fueled with natural gas or syngas, CO 2capture can be implemented at different points, for example, after the fuel cell (“post‐anode capture”). Alternatively, H 2can be produced by reforming/partial oxidation of natural gas or coal gasification upstream the fuel cell and CO 2can be removed after syngas is shifted by means of physical solvents, membranes, or adsorbents – “pre‐anode CO 2capture,” similar to pre‐combustion.
Fuel cells generally operate with an approach that is similar to the “oxyfuel” concept, oxidizing fuel with oxygen extracted from air while generating power and releasing concentrated effluents at the anode outlet ( Figure 1.9). This kind of power cycles generally require an integration with custom‐tailored gas turbine cycles, often operating at unconventional turbine inlet temperatures and pressure ratios, either using natural gas as a fuel or coal through integrated gasification fuel cell (IGFC) concepts. Because most fuel is oxidized in the fuel cell to allow a high CO 2capture efficiency, the fuel cell (FC) generates the majority of the cycle power output. The alternative option offered by MCFCs is shown at the bottom of Figure 1.9, where the fuel cell can operate “draining” CO 2from the cathode inlet stream, receiving the flue gases of a conventional power plant. In this configuration, the fuel cell operates with a post‐combustion approach, although also oxidizing a minor portion of additional fuel with the same “oxyfuel” features discussed above.
The parameters affecting the selection of operating conditions of the SOFC/MCFC are stack size, heat transfer rate, voltage output and cell life, load requirement, and cost. The main operating conditions are pressure, fuel utilization factor at the anode and O 2/CO 2utilization factor (for SOFC and MCFC cases, respectively) at the cathode, voltage, current density, and temperature. The optimization of the process configuration in conjunction with optimal operating parameters is critical to minimize stack degradation, which directly impacts the performance and life of the FC.
Currently, the main challenges for stationary fuel cells are cost and cell durability. For the IGFC system, the gas cleaning process adds another energy barrier to its power generation.
1.2.5.1.1 Solid Oxide Fuel Cells (SOFCs)
Adams et al. [40] divided SOFC systems for CO 2capture into first‐ and second‐generation systems as a function of the operating pressure of the SOFC. Low‐pressure, first‐generation SOFC systems are the most promising option for SOFC commercialization at large scale (100 MW or greater) in the short term. Several process configurations and design options are possible ( Figure 1.10), although those generally follow the same pattern and offer some flexibility to select the optimum combination of variables such as gas clean‐up/reforming, water gas shift (WGS), CO 2capture technology, and heat recovery.
Second‐generation SOFC systems are high‐pressure SOFCs with separate streams for the anode and cathode exhausts. This arrangement promotes the use of an SOFC system that captures and compresses CO 2at significantly reduced costs and minimum complexity via “pre‐anode” and/or “post‐anode” capture.
In the pre‐anode CO 2capture process, syngas is generated at high pressure through high pressure coal gasification or by reforming the natural gas available from a natural gas pipeline at high pressure. Similar to the above cases, the syngas can be optionally shifted using the WGS reaction, creating a stream of steam, H 2, and CO 2. Up to about 90% of the CO 2can then be recovered from the syngas (or shifted syngas) using absorption or adsorption technologies.
Читать дальше













