In 2017, J.M. Serra, C. Kjølseth, and colleagues reported an outstanding breakthrough regarding the production of pure hydrogen from methane. A protonic membrane reformer (PMR) electrochemically driven capable of realizing four process steps simultaneously, achieving near‐zero energy loss (see Figure 3.8a). The reformer is made out of a dense proton‐conducting layer of BaZr 0.8–x–yCe xY yO 3−δ(BZCY), sandwiched between two porous electrodes (BZCY and Ni) [122]. Figure 3.8b represents the PMR concept, where methane is reformed with steam (H 2O) to produce hydrogen that is subsequently transported through the membrane and finally compressed as a consequence of the applied voltage. The study reported full methane conversion permeating 99% of the produced hydrogen at 800 °C. The permeated hydrogen was electrochemically compressed up to 50 bar.
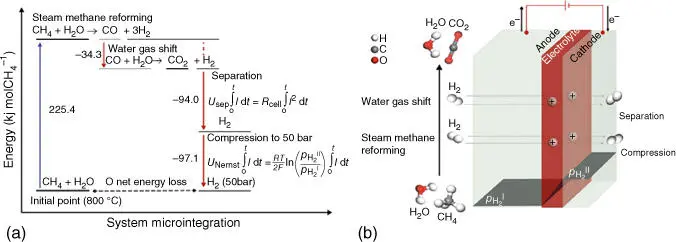
Figure 3.8(a) Representation of the four chemical steps. (b) Protonic membrane reformer.
Source: Malerød‐Fjeld et al. [122].
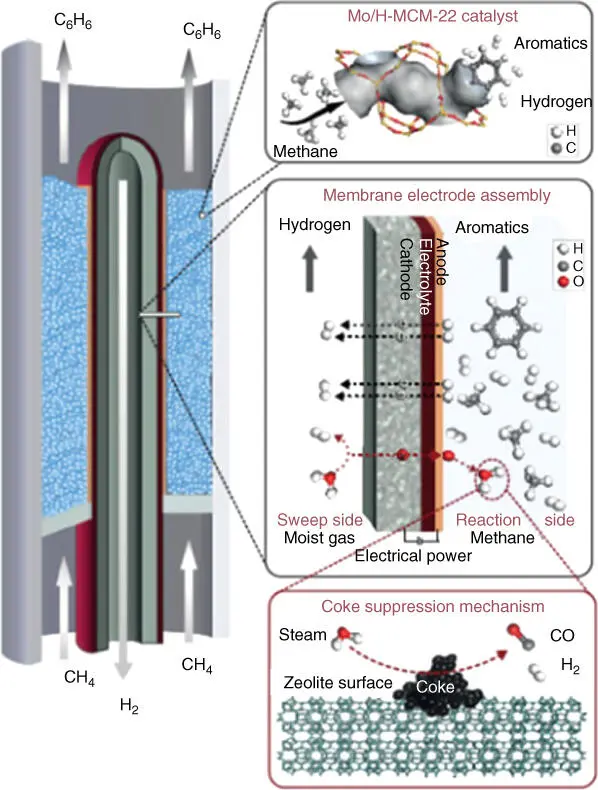
Figure 3.9Co‐ionic catalytic membrane reactor.
Source: Morejudo et al. [125].
3.5.3.2 Methane Dehydroaromatization
A clear example of the contribution of membranes toward chemical production and process intensification is the direct conversion of methane‐containing sources, i.e. natural gas or biogas, into valuable petrochemicals using MDA reaction [123]. This reaction, normally carried out at 700 °C, suffers from some drawbacks, and rapid catalyst deactivation occurs because of the accumulation of polyaromatic‐type coke, impeding the access to the catalyst‐active sites and the limitation imposed by thermodynamics of the per‐pass conversion to aromatics [124]. In order to overcome the abovementioned disadvantages, a recent publication reports a CMR for MDA process intensification [125]. The system is composed of an electrochemical BaZrO 3‐based tubular membrane that exhibits proton and ion conductivity. Figure 3.9depicts a representation of the MDA reaction and the different components/materials that form the CMR.
In this case, methane is converted to benzene and hydrogen using a Mo/H‐MCM‐22 catalyst. Protons are transported to the sweep side, whereas oxide ions are transported to the reaction chamber and react with H 2to form water that will subsequently react with coke to form CO and H 2, enhancing catalyst stability. The authors observed that the electrochemically driven simultaneous extraction and injection of proton and oxide ions, respectively, allows obtaining high aromatic yields while drastically reducing the catalyst deactivation rate by coking.
3.6 Conclusions and Final Remarks
Membrane technologies are striking as strong candidates to tackle climate change by presenting potential and effective solutions for capturing CO 2and also for performing its conversion toward highly valuable chemicals. In order to set the different available options within the membrane field, different types of membranes have been summarized in this chapter, together with the outline of the main strategies and novel solutions toward the CO 2valorization.
Therefore, the main issues concerning the most promising concepts, their particularities and properties, as well as their advantages and drawbacks when considering their industrial application have been presented. Also, a discussion on how these options can be integrated into the existing engineering layouts has been performed. This led to the presentation of some of the most advanced developments up to date, with an extended description of their operating concepts, and how their implementation can help to the effective valorization of CO 2while reducing the emission problem.
It is clear that many research groups are working to develop new materials to enhance these processes; however, more efforts are necessary from the research and exploitation point of view of this technology in order to promote membranes in an industrial way.
1 1 IEA (2020). CO2 Emissions from Fuel Combustion: Overview. Paris: IEA. https://www.iea.org/reports/co2-emissions-from-fuel-combustion-overview.
2 2 Yu, X., Yang, J., Yan, J., and Tu, S. (2015). Membrane technologies for CO2 capture. In: Handbook of Clean Energy Systems (ed. J. Yan), 1–13. Wiley.
3 3 Remiro‐Buenamañana, S. and García, H. (2019). ChemCatChem 11 (1): 342–356.
4 4 Mulder, J. (2013). Basic Principles of Membrane Technology. Netherlands: Springer.
5 5 Mohanty, K. and Purkait, M.K. (2011). Membrane Technologies and Applications. CRC Press.
6 6 Paidar, M., Fateev, V., and Bouzek, K. (2016). Electrochim. Acta 209: 737–756.
7 7 Li, H., Caravella, A., and Xu, H.Y. (2016). J. Mater. Chem. A 4 (37): 14069–14094.
8 8 Amano, M., Nishimura, C., and Komaki, M. (1990). Effects of high concentration CO and CO2 on hydrogen permeation through the palladium membrane. Mater. Trans. JIM 31: 404–408.
9 9 O'Brien, C.P. and Lee, I.C. (2017). J. Phys. Chem. C 121 (31): 16864–16871.
10 10 Brunetti, A. and Fontananova, E. (2019). J. Nanosci. Nanotechnol. 19 (6): 3124–3134.
11 11 Pomilla, F.R., Brunetti, A., Marcì, G. et al. (2018). ACS Sustainable Chem. Eng. 6 (7): 8743–8753.
12 12 Mason, E.A. (1991). J. Membr. Sci. 60 (2): 125–145.
13 13 Mitchell, J.K. (1995). J. Membr. Sci. 100 (1): 11–16.
14 14 Koros, W.J. and Fleming, G.K. (1993). J. Membr. Sci. 83 (1): 1–80.
15 15 Wijmans, J.G. and Baker, R.W. (1995). J. Membr. Sci. 107 (1): 1–21.
16 16 Baker, R.W. (2012). Membrane Technology and Applications, 3e. Wiley.
17 17 Tanaka, K., Kita, H., Okano, M., and Okamoto, K.‐i. (1992). Polymer 33 (3): 585–592.
18 18 White, R.P. and Lipson, J.E.G. (2016). Macromolecules 49 (11): 3987–4007.
19 19 Han, Y. and Ho, W.S.W. (2018). Chin. J. Chem. Eng. 26 (11): 2238–2254.
20 20 Zhao, L., Weber, M., and Stolten, D. (2013). Energy Procedia 37: 1125–1134.
21 21 Yave, W. and Car, A. (2011). Chapter 6: Polymeric membranes for post‐combustion carbon dioxide (CO2) capture. In: Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications (eds. A. Basile and S.P. Nunes), 160–183. Woodhead Publishing.
22 22 Ockwig, N.W. and Nenoff, T.M. (2007). Chem. Rev. 107 (10): 4078–4110.
23 23 Weigelt, F., Escorihuela, S., Descalzo, A. et al. (2019). Membranes 9 (4): 51.
24 24 Escorihuela, S., Tena, A., Shishatskiy, S. et al. (2018). Membranes 8 (1): 16.
25 25 He, X. (2016). Membranes for natural gas sweetening. In: Encyclopedia of Membranes (eds. E. Drioli and L. Giorno), 1266–1267. Berlin, Heidelberg: Springer Berlin Heidelberg.
26 26 Robeson, L.M. (1991). J. Membr. Sci. 62 (2): 165–185.
27 27 Robeson, L.M. (2008). J. Membr. Sci. 320 (1): 390–400.
28 28 Kim, S. and Lee, Y.M. (2013). Curr. Opin. Chem. Eng. 2 (2): 238–244.
29 29 Budd, P.M., Msayib, K.J., Tattershall, C.E. et al. (2005). J. Membr. Sci. 251 (1): 263–269.
30 30 Park, H.B., Jung, C.H., Lee, Y.M. et al. (2007). Science 318 (5848): 254.
31 31 Perrin, N., Dubettier, R., Lockwood, F. et al. (2015). Appl. Therm. Eng. 74: 75–82.
32 32 Lupion, M., Alvarez, I., Otero, P. et al. (2013). Ghgt‐11 37: 6179–6188.
33 33 Monne, J. and Prinet, C. (2013). Ghgt‐11 37: 6444–6457.
34 34 Smart, S., Lin, C.X.C., Ding, L. et al. (2010). Energy Environ. Sci. 3 (3): 268–278.
Читать дальше














