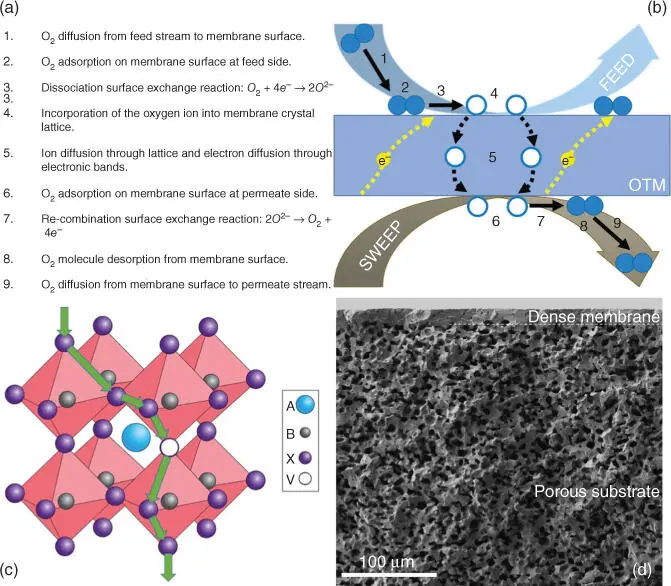
Figure 3.4(a, b) Steps involved in the oxygen permeation through an oxygen transport membrane and (c) representation of the oxygen anion diffusion through the oxygen vacancies present in a perovskite's crystal lattice and (d) fracture cross.
Source: (c) Bouwmeester and Burggraaf [38].
For thinner membranes and for temperatures typically lower than 800 °C, J (O 2) does not depend on O 2−bulk diffusion but on oxygen surface exchange kinetics. These surface reactions involve a number of steps that may include O 2adsorption, O 2dissociation into O 2−and electrons, charge transfer, surface diffusion of intermediate species, and finally O 2−incorporation into the material crystal lattice [39, 40]. A general expression for describing J (O 2) can be obtained by assuming linear kinetics of the relevant rate laws when both surface exchange reactions and bulk diffusion are governing the O 2permeation and then J (O 2) [41] can be expressed by means of the following expression:
(3.5) 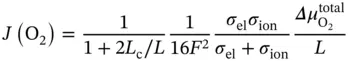
where 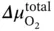 is the total O 2chemical potential difference across the membrane.
is the total O 2chemical potential difference across the membrane.
Therefore, the O 2permeation of an OTM depends on temperature, membrane thickness, p O 2gradient between the two membrane sides, and the MIEC properties of the considered material. Then, higher O 2fluxes can be achieved by operating at higher temperatures and larger p O 2gradients (use of pressurized feeding or high vacuum or sweeping flows), by using thinner membranes (material brittleness will require the use of porous supports for ensuring mechanical stability as depicted in Figure 3.4d where a thin supported membrane is shown) and by selecting materials with high ionic and electronic conductivities.
The most considered materials for being used as OTMs are those with perovskite and fluorite crystal structures [38]. In addition to these materials, other compounds that also exhibit interesting properties are pyrochlore (A 2B 2O 7), brownmillerite (A 2B 2O 5), Ruddlesden–Popper series (A n+1B nO 3n+1), orthorhombic K 2NiF 4‐type structure materials, and Sr 4Fe 6−xCo xO 13[42–45]; nevertheless, the performance of this materials is very low in comparison with fluorites and perovskites. Among the mentioned structures, the perovskite with composition Ba 0.5Sr 0.5Co 0.8Fe 0.2O 3−δ(BSCF) is that presenting the highest O 2permeation up to date, achieving an O 2production of up to 67.7 ml min −1cm −2at 1000 °C for a 70 μm thick membrane [46]. Nevertheless, the unpractical stability under certain environments (especially when exposed to CO 2atmospheres) makes BSCF‐based membranes unsuitable for most of the industrial applications if a direct contact between flue gases and membranes is considered. Fluorites, on the contrary, exhibit outstanding chemical and mechanical stability when exposed to oxyfuel and reaction atmospheres, but their low electronic conductivity averts them for being considered for practical applications because of the low O 2permeation performance. One solution is then the use of dual‐phase structures consisting of a mixture of ionic‐conductive and electronic conductive materials. These materials with good O 2permeation and stability when subjected to harsh environments have attracted a lot of interest within the past years in studies focused on oxyfuel applications [47–51], achieving interesting O 2fluxes of c. 3 ml min −1cm −2at 925 °C under full CO 2environments [51].
3.3.2 Application Concepts of OTMs for Carbon Capture and Storage (CCS)
As already stated for the application of CCS strategies, OTMs are considered as O 2supply units for the conduction of combustion processes. In that way, an OTM module is integrated within an industrial process following approaches that use waste heat streams for heating up the module to its operation temperature (typically 800–900 °C). Therefore, the operation and integration can be done according to two different configurations, known as 3‐end and 4‐end modes ( Figure 3.5a,b). These modes differ mainly on the number of streams connected to the OTM module. As can be seen in Figure 3.5a, the 4‐end mode uses a fraction of the flue gas (mainly consisting of CO 2and H 2O) from the burner for heating the module and for performing O 2separation as a sweeping agent. As already pointed out, the recirculation of flue gases increases the plant efficiency, also reducing O 2production costs due to the highly synergetic thermal integration of OTM modules in the process. Differently, in the 3‐end mode, any contact between flue gases and membrane materials is avoided. In this configuration, O 2is extracted using a vacuum pump at the permeate side, thus obtaining a pure O 2stream that is used for combusting the fuel. The thermal integration is done by heating the air feed stream with heat exchangers where the heat is transferred from the exiting flue gases. This operation mode is considered for the cases where membrane material cannot operate under flue gas atmospheres. The selection of one or another configuration will influence parameters such as module design (especially the required membrane surface area) and plant layout, also determining the overall plant efficiency [52, 53]. Nevertheless, there is no preferred mode for conducting the OTM module integration. This can be observed in the OTM developments carried out by Praxair [54] and Air Products and Chemicals Inc. [55] (the most advanced up to date), which consider 4‐end and 3‐end operation modes, respectively.
3.3.3 Existing Developments
Currently, the R&D efforts are mainly focused on the development of new materials and membrane architectures with better performance and stability, as well on the search of new applications. All these developments are being carried out at laboratory scale; nevertheless, there are companies and institutions that are advancing in the upscaling of OTM concepts, with significant progresses in integrating ceramic membrane modules in industrial environments. Among all, the most advanced progresses have been achieved by Praxair and Air Products [56, 57], with the construction of demonstrative plants with OTM technology at high Technology Readiness Levels (TRL). These companies have worked for more than 20 years in the development of industrial‐scale OTM modules and integrated gasification combined cycle (IGCC) systems with ceramic membranes for O 2separation.
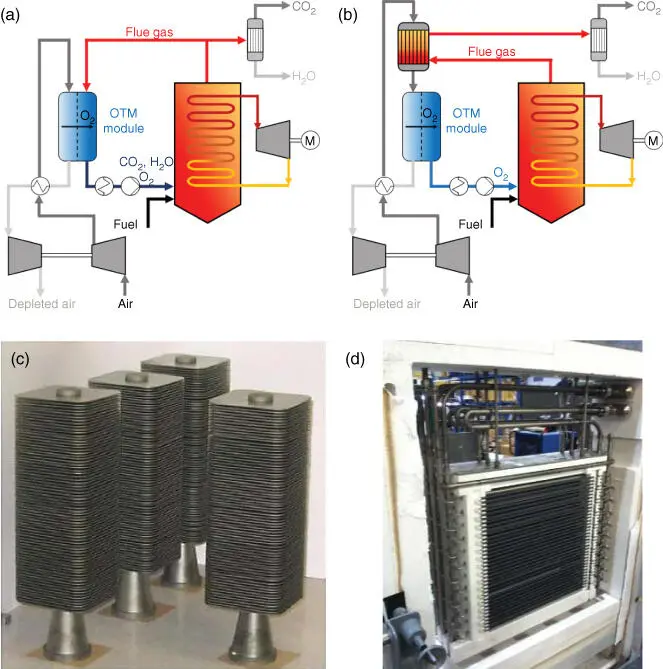
Figure 3.5Simplified process layouts for oxygen permeating membrane modules integrated in oxyfuel power plants following (a) 4‐end and (b) 3‐end mode approaches, (c) Air Products' planar stacks, and (d) combined system steam reformer‐OTM‐ATR developed by Praxair.
Source: Linde.
With regard to Air Products, the most advanced developments consisted of an intermediate scale testing with a capacity of 100 temperature programme desorption (TPD) O 2(corresponding to an IGCC output of 12 MW) [58] and a membrane vessel consisting of several 1 TPD O 2OTM modules (as those shown in Figure 3.5c), with a total production of 2000 TPD O 2. Despite that Air Products developments are the most advanced in terms of integration and demonstration, performance, and TRLs, they have been apparently abandoned since 2015 because of a company structure reorganization.
Читать дальше


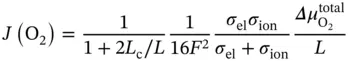
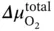 is the total O 2chemical potential difference across the membrane.
is the total O 2chemical potential difference across the membrane.











