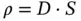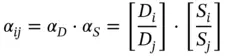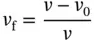These proposed actions for enabling the CO 2capture and conversion can be implemented by applying the so‐called membrane technology. Membranes are materials acting as semipermeable barriers between two different phases, and because of their intrinsic properties, they are able to selectively transport a particular component ( Figure 3.1). The different characteristics between retentate and permeate will work as the driving force and the membrane properties will set the separation degree. The transport mechanism of the membrane will be determined by the membrane composition, structure (homogeneous or heterogeneous), morphology, origin (natural or synthetic), and operation conditions [4]. Most of the membranes are based on the pore dimension principle, where the pore size will act as the cutoff of the compounds that are able to pass through the membrane, blocking in the retentate larger species [5]. Hence, as a general classification, membranes can be divided into microporous membranes and dense membranes. In addition, selective membranes without electrostatic interaction make up a significant proportion of membrane technology. A selective membrane only allows certain molecules to be transported along the membrane to the permeate side. Finally, for ion‐selective membranes, the transport of ions is ascribed to the compensation of charges in the system by charge‐carrying species [6]. Furthermore, membranes possess some advantages such as low energy consumption, mild reaction conditions, ability to continuously perform separation processes, generally facile scaling up, and among others [4].
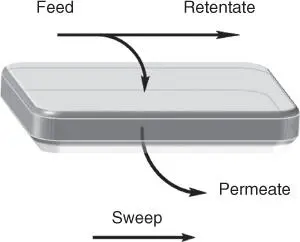
Figure 3.1Gas transport through a membrane.
This chapter will take the reader along the most relevant membranes for the aim of CO 2carbon/capture applications, and these can be divided into polymeric, ionic, protonic, and electrochemical membranes. Metallic membranes are not included in this chapter because of several reasons, such as their production costs and CO poisoning. Some studies report the retarding effects of CO on H 2permeation through Pd‐based membranes. This detriment on H 2diffusion through the active sites of Pd became more significant at temperatures around 250 °C and/or higher CO concentration [7–9]. In the majority of industrial applications for CO 2capture or storage, CO may appear as a subproduct. Hence, the use of Pd membranes for H 2/CO 2separation processes may be limited.
Regarding the different applications, membrane reactors have demonstrated to be promising candidates to tackle climate change, decreasing the levels of CO 2by using it in capture–conversion technologies to obtain valuable chemicals [10, 11]. The present chapter describes a range of approaches toward CO 2capture–conversion in the context of catalytic membranes. Because of the extension of the field, this chapter will briefly summarize different types of gas separation and gas absorption membranes that are currently being investigated for CO 2applications.
Polymer materials for gas separation membranes have been widely used for different applications in the past three decades. Although the first recorded description of a semipermeable membrane was in 1748 [12], followed by the observation of the permeation of H 2through balloons in 1831 [13], it was not until the late 1970s when several experiments demonstrated the great commercial potential of polymeric gas separation membranes [14].
Polymer membranes are dense (nonporous) membranes, which follow the solution–diffusion model as a transport mechanism [15]. In this model, gas is transported through a dense polymer membrane in three steps: (i) dissolving into the face of the membrane that is exposed to high gas pressure, (ii) diffusion through the polymer, and finally (iii) desorbing from the face of the membrane that is exposed to low pressure. The rate‐limiting step for gas separation polymer membranes is the second step, diffusion of gas through the polymer material [15]. As a consequence, permeability can be expressed as the product of diffusion coefficient and solubility coefficient.
(3.1) 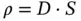
Diffusion coefficient is related to the kinetic terms, and it reflects the mobility of the individual molecules in the membrane material. In other words, it depends on the molecular size of the target gas (step ii). On the other hand, solubility coefficient links the concentration of a component in the fluid phase with its concentration in the membrane polymer phase and reflects the number of molecules dissolved in the membrane material (step i and iii). It depends on molecular interaction; hence, it is an equilibrium term [15].
Regarding the selectivity or separation factor of polymer membranes, it is defined as a product of mobility selectivity, ( D i/ D j) the fraction of the diffusion coefficients of the two gases, the solubility coefficient ( S i/ S j), and the ratio of the solubility coefficients of the two gases.
(3.2) 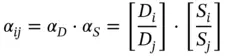
Based on these previous explanations, membrane material is more related to diffusion coefficient, being more affected by the membrane changes, than solubility coefficient. In order to explain the concept in a more visual way, as an example, diffusion and solubility coefficients of four different gases in a group of several related polyimides are plotted against each other. Both coefficients are reasonably well grouped for each gas as it can be shown in Figure 3.2. Thus, for any gas, the difference in diffusion coefficient from the highest value is 100 times bigger than the lowest value, while the deviation in solubility coefficients is only 2–4 times. Changes in polymer chemistry affect both diffusion and solubility coefficients, but the effect on the diffusion coefficient is more meaningful [16]. Diffusion coefficient will differ depending on the polymer nature (rubber or glass). There is a considerable difference between the motion of polymer segments in a malleable rubbery polymer and in a stiff glassy polymer. Consequently, diffusion coefficients of glassy polymer are usually lower than the diffusion coefficients of rubbery polymers, and in addition, diffusion coefficients of glassy polymers decrease faster with increasing permeate molecular size. In other words, the mobility selectivity term for rubbery membranes is smaller than the mobility coefficient of glassy membranes.
Fractional free volume (FFV) parameter is used in order to correlate permeation properties with the structure and chemical properties of the polymers. Permeability factor of gases in glassy polymers are highly dependent on the FFV, which can be defined as the free space that is not occupied by the polymer molecules [18]. FFV is defined by
(3.3) 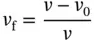
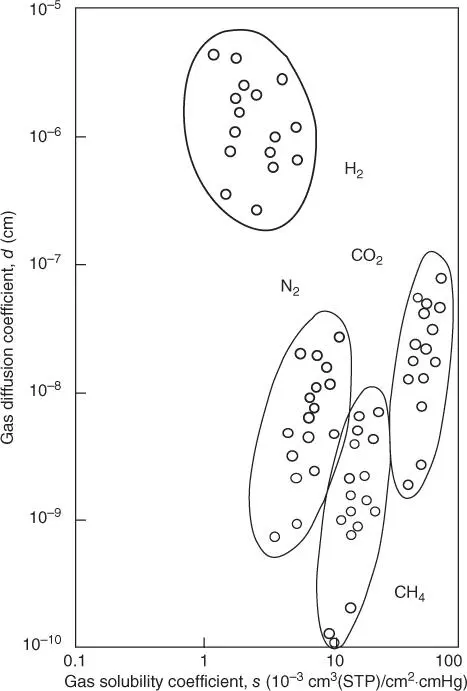
Figure 3.2Diffusion and solubility coefficient for different polyimides.
Source: Adapted from Baker and Tanaka et al. [16, 17].
where v is the specific volume of the polymer (cm 3g −1), i.e. the correlative of the polymer density, and v 0is the volume occupied by the polymer molecules (cm 3g −1). In principle, FFV is the sum of all the spaces between the polymer chains.
Читать дальше