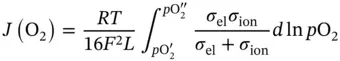Nowadays, polymer membranes for CO 2capture are being intensively investigated, specifically regarding post‐combustion carbon capture (CO 2/N 2), hydrogen purification (CO 2/H 2), and natural gas sweetening (CO 2/CH 4) [19]. In terms of post‐combustion carbon capture, competing with chemical absorption, gas separation membranes appear as a more environmentally friendly solution. As an example, a simulation of two potential polymer membranes, Polyactive® and PVAm/polyvinyl alcohol membranes, was performed for a 600 MW (gross) reference power plant [20]. Other materials, such Pebax, were also investigated for similar purposes, regarding CO 2capture in post‐combustion processes [21].
Regarding H 2purification, the predominant technology is steam methane reforming (SMR), where H 2is obtained together with, mainly, CO 2(15–20%) among other gases [22]. Polymeric membranes not only show advantageous properties, such as ease of processing or low cost, but also would be more accessible for large‐scale applications over other types of membranes. Consequently, and regarding high‐temperature processes, different polyimides such as 6FDA–6FpDA and Matrimid, with outstanding H 2/CO 2separation properties at temperatures up to 270 °C, have been recently reported [23, 24].
With regard to natural gas sweetening, or in other words, CO 2removal from natural gas, membrane systems show greater potential than conventional chemical absorption because they have main advantages such as low cost, environmentally friendly technologies, and process flexibility [25]. Several commercial membranes are available for CO 2elimination from natural gas. The most common representative materials for CO 2removal are cellulose acetate/triacetate and polyimide [25].
In order to study the properties of different membrane materials, and to be able to compare different polymer materials, Robeson published several charts, the first time in 1991 and the latest in 2008, about membrane permeability as a function of selectivity. These charts are called upper bound correlation [26, 27]. These experimental data are obtained from single gas tests at 30 °C and 1 bar. This indicates that permeabilities were measured using pure gas tests, and selectivities were obtained from the ratio of the pure gas permeabilities. This gives ideal selectivities, despite the fact that industrial processes of gas separation membranes are commonly performed with gas mixtures. Nevertheless, it is possible to extrapolate to industrial applications, this is because, in principle, if gases do not interact with the membrane material, the difference between single gas selectivity and gas mixture selectivity will be small. Consequently, in gas mixtures, molecules that possess high‐solubility coefficients will be sorbed enough by the membrane material to affect the other gas permeabilities.
Hence, upper bound correlations are described for several gas pairs. Regarding CO 2separation, two different gas pair (CO 2/N 2and CO 2/CH 4) are studied, CO 2/CH 4being the second most investigated gas pair for membrane separation. In Figure 3.3a,b, a large number of polymers have been shown, which are mostly placed between a selectivity of 2–100 and a permeability of 0.1–1000. In addition, two black lines are identifying the prior upper bound and the present upper bound. Any polymer that surpasses the present upper bound can be considered as the most promising polymer for CO 2separation.
As an example, PIM‐1 with a permeability of 2300 Barrer and a selectivity of 18.4, or PIM‐7 with a permeability of 1100 Barrer and a selectivity of 17.7 [28], is considered as a ladder polymer for the gas pair CO 2/CH 4, which surpasses the upper bound [29]. Thermally rearranged polymers have been recently published, which exhibit outstanding improved separation characteristics, although the present upper bound is determined without these data [30], see Figure 3.3a.
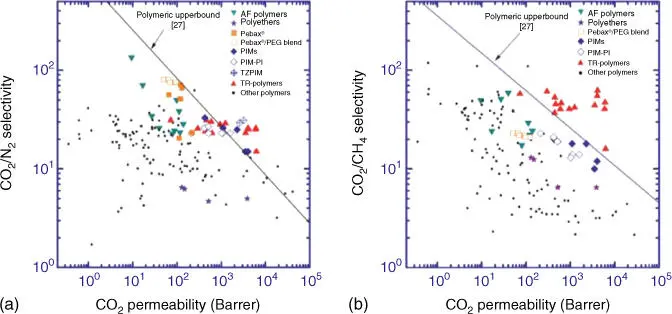
Figure 3.3Upper bound correlation for (a) CO 2/CH 4separation and (b) CO 2/N 2separation in 2008.
Source: Kim and Lee [28].
3.3 Oxygen Transport Membranes for CO2 Valorization
The application of strategies for capturing the CO 2from combustion processes is of great importance for effectively implementing CO 2conversion approaches and thus reducing the impact of industrial activity regarding CO 2emissions. Oxyfuel technology is one of the most considered options for conducting the implementation of this capture and sequestration of CO 2from exhaust gases, as well as for improving the efficiency of the aforementioned industrial processes [31–33]. The oxyfuel approach applied to combustion processes consists of burning the fuel with a pure or O 2‐rich stream, thus obtaining mainly CO 2and H 2O as products. Consequently, CO 2sequestration can be easily carried out with a simple separation step.
Nevertheless, oxyfuel technology is still far from being implemented in the most of targeted industrial processes. The main reason is related to the O 2supply, which is not economically feasible for those applications. Currently, almost all the O 2is produced by cryogenic distillation of air. This process, which is operated at very low temperatures and high pressures, requires from high energy and large production plants that avert its consideration for O 2on‐site production in small‐ and medium‐scale installations. An appealing solution for implementing an O 2supply system in such applications can be performed by considering oxygen transport membranes (OTMs). OTMs are ceramic membranes consisting of metallic oxides with the ability of diffusing O 2−ions through the oxygen vacancies present in their crystal lattice. This is due to the mixed ionic–electronic conductivity (MIEC) of these materials at high temperatures (>600 °C), thus allowing O 2separation with 100% selectivity from a high p O 2feed stream (pressurized air) to a low p O 2sweep stream (vacuum or recirculated flue gas stream). Furthermore, waste heat streams generated in high‐temperature processes in several industries requiring from O 2supply for conducting combustions (e.g. cement plants, ceramic and glass production, and power plants) can be used for heating the OTM modules up to their operating temperature. This thermal integration can then result in a significant increase in plant efficiency and in a reduction in O 2production costs, which can be lowered up to 35% with respect to conventional cryogenic distillation [34].
3.3.1 Oxygen Transport Membrane Fundamentals
The ceramic materials exhibiting MIEC properties that are considered for constituting OTMs because of their good O 2permeation properties are known since the 1970s [35–37]. OTMs typically consist of dense layer(s) of multimetallic oxides comprising alkali, alkali earth, rare earth, or transition metals together in the same crystalline structure. The O 2permeation through a ceramic membrane comprises several steps, which are depicted in Figure 3.4a,b.
As can be seen in Figure 3.4a,b, O 2permeation depends on two main processes: oxygen bulk diffusion (step 5) and oxygen surface reactions (steps 2–4 and 6–8). Figure 3.4c depicts O 2‐bulk diffusion through oxygen vacancies in the material's cristal lattice. Typically, O 2permeation is described by using Wagner's equation (Eq. (3.4))
(3.4) 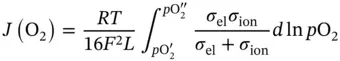
where J (O 2) is the O 2flux through the membrane, R is the gas constant, T is the temperature, F is Faraday's constant, L is the membrane thickness, p O 2″ and p O 2′ stand for the O 2partial pressures at feed and permeate sides, respectively, and σ eland σ ionare the electronic and ionic conductivities of the considered membrane material, respectively. Wagner's equation describes the O 2permeation through the material bulk (step 5 in Figure 3.4a,b), when the membrane is thicker than the value known as the characteristic thickness L c[38], which is distinctive of every material.
Читать дальше