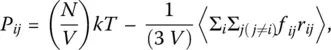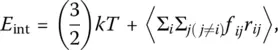The third useful information on short‐range order is the “bond angle distribution” (BAD), because the nature of the existing polyhedral units can be ascertained from oxygen–cation–oxygen angles. For SiO 2and silicate glass, the simulated peak position is, for instance, found near 109.47° in O─Si─O angle distributions, which of course reflects the presence of Si within SiO 4tetrahedra. Likewise, the peak of O─B─O angles in B 2O 3lies around 120°, in harmony with the formation of BO 3triangles ( Figure 7). As for the B─O─B distribution, a significant amount of angles with 120° reveals the presence of boroxol rings ( Figure 7) because, without boroxol rings, the B─O─B distribution should be around 129° as observed in B 2O 3crystal [6]. In this respect, the interest of MD simulations stems from the fact that there is no direct method for determining the BAD experimentally – just a possibility to estimate roughly average values from X‐ray or neutron diffraction data. These simulated cation–oxygen–cation BADs are specially valuable to probe local structural changes with either temperature or pressure in single‐component glasses such as SiO 2, B 2O 3, P 2O 5, or GeO 2.

Figure 7 Bond angle distribution in simulated B 2O 3glass.
The fourth information of interest is the “torsion angle distribution” (TAD), to which particular attention is paid for polymers whose structure and properties are significantly affected by the twisted arrangement of side chains. Again, B 2O 3glass illustrates the relevance of this distribution to inorganic glasses because of the steric problem raised by the interconnection of BO 3units and boroxol B 3O 6rings ( Figure 8). In this case, the calculated TAD suggests different connecting geometries between BO 3–BO 3linkages and B 3O 6–B 3O 6units: the former are preferentially oriented in a perpendicular direction, which means that connected BO 3units do not lie in the same plane, whereas the latter are oriented in the same direction, which means that B 3O 6units do lie in the same plane [6]. For SiO 2glass, no such distinction is of course found, but MD simulations do suggest that a torsional transformation takes place between neighboring SiO 4tetrahedra at elevated temperature, in analogy with the structural changes associated with the α – β transition in cristobalite [17]. In addition, it has been suggested that the abrupt rotation of Si─O─Si equivalent to torsion movement between two SiO 4tetrahedra is the cause of anomalous thermomechanical properties in silica glass [17].
Medium‐range order is difficult to study either experimentally or through numerical simulations. The size distribution of rings made up of cations and oxygen atoms is in particular an important parameter when investigating geometrical features in the 5–15 Å range. Usually a ring is characterized by the number of its network‐forming cations, which can be derived from the calculated atomic coordinates as shown in Figure 9for simulated B 2O 3and SiO 2glasses. Compared with cristobalite, tridymite, and quartz, whose ring sizes are 6, 6, and 6 and 8, respectively, simulated SiO 2glass shows a broad distribution around 6. It has been speculated that the existence of sizes of odd‐numbered rings are characteristic of disordered structures and might impede glass crystallization, because fivefold rotational symmetry does not exist in crystals where four‐, six‐, and eight‐membered rings are primarily observed. In B 2O 3glass, which is extremely reluctant to crystallize, the existence of B 3O 6units indeed causes the presence of a peak at 3 in the ring statistics.
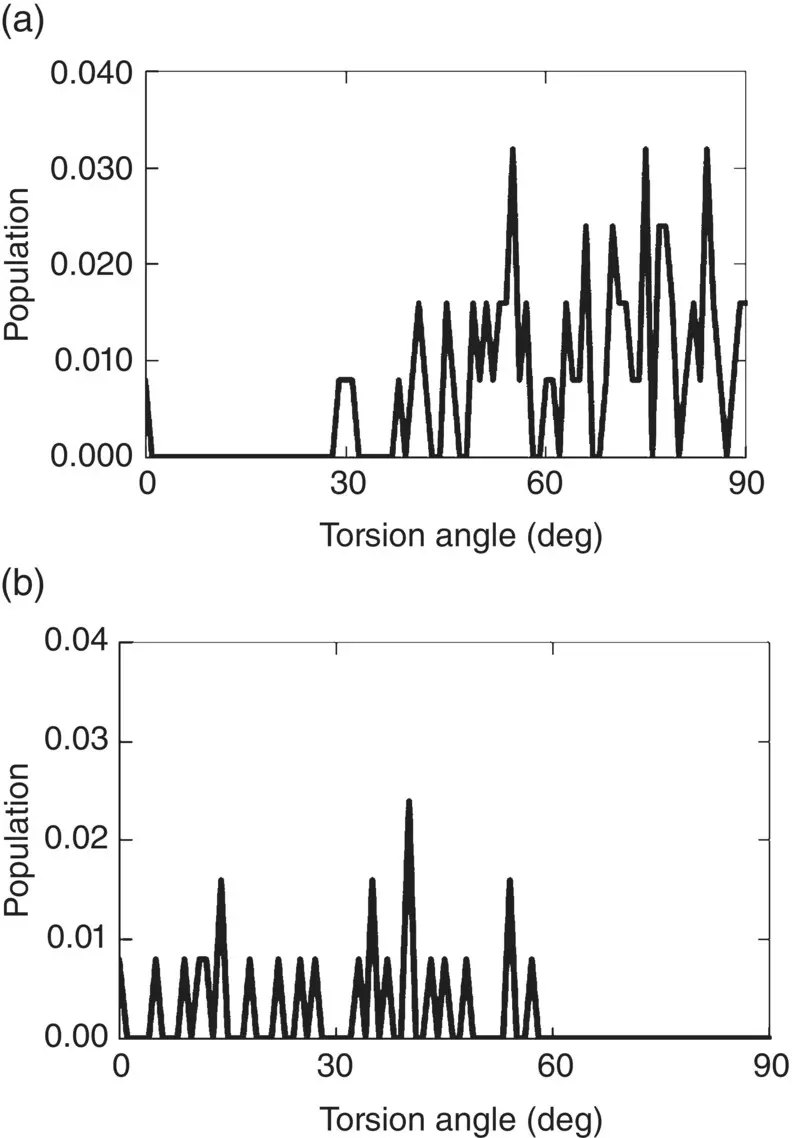
Figure 8 Torsion angle distribution in simulated B 2O 3glass between BO 3and BO 3units (a) and between B 3O 6and B 3O 6units (b) [6]. Data sampled at 0 K to prevent peaks from being blurred by atomic vibrations.
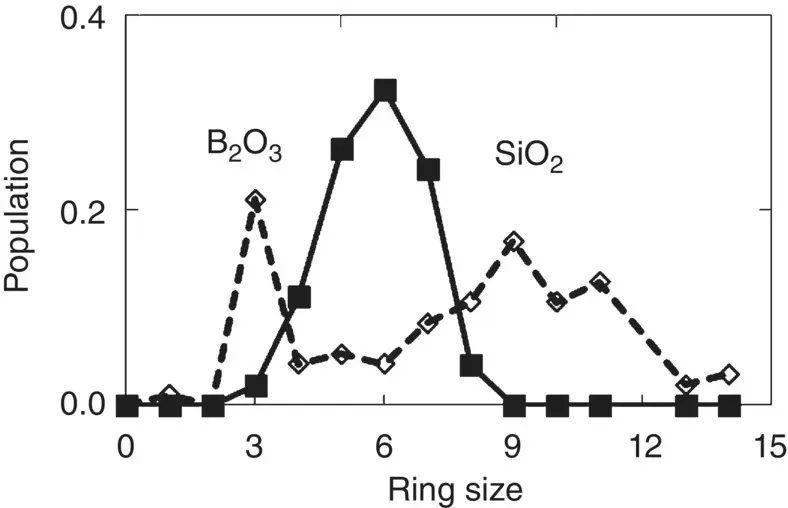
Figure 9 Ring size distribution in simulated B 2O 3and SiO 2glasses [11]. Data sampled at 0 K.
Of more general relevance is the vibrational density of states (VDOS). One can calculate it by solving the eigenvalue problem once the curvature of the energy surface around the stable configuration is obtained. The VDOS for simulated B 2O 3glass is shown in Figure 10, where the peaks labeled A, B, and C represent the vibrations associated with independent BO 3units observed in crystalline B 2O 3,where B 3O 6units are absent, the breathing mode of B 3O 6units observed in the crystal of metaboric acid comprised of B 3O 6unit, and the vibrations of B 3O 6 + nunits with several BO 4tetrahedra comprised in rings [6], respectively. The VDOS can thus provide important structural information in terms of cooperative motion of structural units.
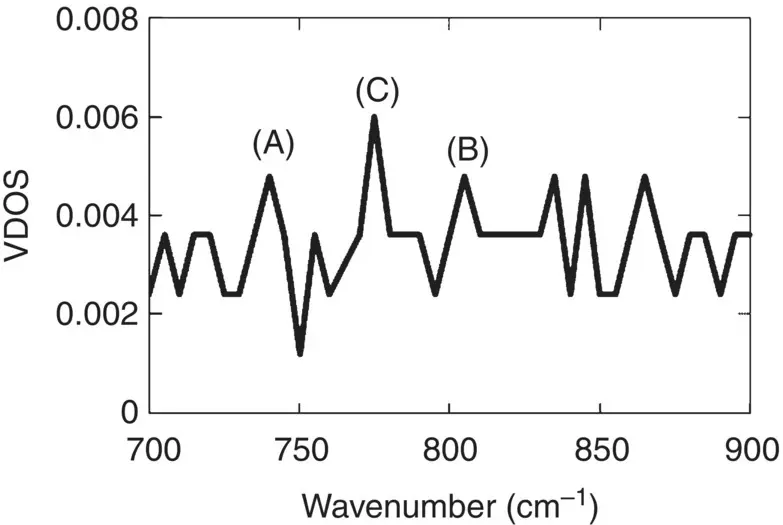
Figure 10 Vibrational density of states in simulated B 2O 3glass [6]. See text for the assignments of the peaks labeled A, B, and C.
There is another geometrical method relying on the so‐called “Voronoi diagram” (e.g. [18]). It is largely employed for monatomic system for which partitioning three‐dimensional space is simple and easy when the calculated atomic coordinates obtained by atomistic simulations are used to delineate the portion of space assigned to every atom. These “Voronoi polyhedra” are then characterized by their numbers of faces and corners whose distributions change as positional relationships vary in the glass structure. The other geometrical method is called the analysis of “bond orientational order.” The order parameter that is rotationally invariant can be calculated with spherical harmonic functions. This order parameter has been used to investigate a local icosahedral order chiefly for monatomic system, because its value can discriminate geometrical differences between FCC, HCP, icosahedral, and BCC clusters (e.g. [18]).
In summary, atomistic simulations provide a key to explaining the concept of “modified random network theory [10]” in alkaline silicate glass [10] or the existence of “super‐structural units [19]” in B 2O 3glass [6]. However, new analytical methods are required to understand medium‐range order in more detail.
5.4 Structure‐related Properties
Important thermodynamic properties can be calculated once numerical simulations have yielded atomic configurations and velocities. The pressure is, for example, calculated from
(24) 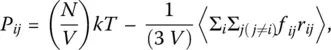
where the bracket indicates an equilibrium time average and N , V , T , f ij, and r ijare as usual the number of atoms, cell volume, temperature, and pair force and distance between atoms i and j , respectively.
The internal energy ( E int) is
(25) 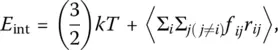
Читать дальше