4 Delaying intervention of infected pancreatic necrosis is associated with better outcomes compared with an early approach [13,14]. Therefore, intervention should be delayed as much as possible, at least four weeks from onset of the disease, when WON with mature wall has developed.
5 An endoscopic approach has been established as a valid and probably safer alternative to the minimally invasive surgical approach for the step‐up treatment of patients with infected (peri)pancreatic necrosis [15–17].
Prevention of Infection of (Peri)pancreatic Necrosis
Intestinal bacterial translocation is generally accepted as the main source of infection of pancreatic necrosis [18]. In fact, intestinal permeability is increased and splanchnic perfusion is impaired in severe acute pancreatitis [19,20]. In addition, the bacteria infecting pancreatic necrosis are mainly of intestinal origin [21]. Since antibiotic prophylaxis has failed to provide any benefit in acute necrotizing pancreatitis [4–9] and because it is definitely not recommended in clinical practice, avoiding intestinal bacterial translocation is the main aim in the prevention of infection of pancreatic necrosis.
Enteral nutrition is one of the pillars in the management of severe acute pancreatitis. This not only provides nutrients to a severely ill patient, but also maintains intestinal barrier function. Enteral nutrition improves luminal gut nutrition, enhances splanchnic blood flow, and may stimulate enteral motility, thus reducing the risk of intestinal bacterial overgrowth and bacterial translocation [22,23]. This explains, at least partly, why enteral nutrition is superior to parenteral nutrition in patients with severe acute pancreatitis in terms of infection of pancreatic necrosis, septic complications, and death [24,25].
Enteral nutrition can be delivered through a nasogastric or a nasojejunal tube [26,27]. Based on the similar efficacy and safety of both routes, nasogastric feeding is generally preferred as the first step in clinical practice. Nasojejunal feeding is mainly reserved for patients with gastric outflow obstruction and those with intolerance to gastric feeding.
Early initiation of enteral feeding was generally recommended in clinical practice for patients with predicted severe acute pancreatitis. However, recent clinical trials have shown that early feeding is not associated with a reduction in infection, persistent organ failure, or mortality [28,29]. Based on this, enteral feeding can be delayed and given on demand to patients who are unable to tolerate oral refeeding after 72 hours from onset of acute pancreatitis.
Diagnosis of Infected (Peri)pancreatic Necrosis
The presence of fever early in acute pancreatitis is usually secondary to the release of inflammatory mediators. Later, the development of clinical and laboratory markers of sepsis in the absence of any extrapancreatic infection (respiratory, urinary, venous catheter) is the basis for the diagnosis of infected pancreatic necrosis. The presence of gas within the (peri)pancreatic area on computed tomography (CT) confirms the diagnosis ( Figure 14.1). In the absence of pancreatic gas bubbles, which occurs in up to 60% of patients [30], fine needle aspiration (FNA) may be required to confirm the diagnosis of infection within the pancreatic necrosis. However, culture of FNA samples produces false‐negative results in about 25% of cases and frequently does not modify the therapeutic approach in patients with sepsis [30]. In our center, FNA for culture and antibiotic resistance testing is only performed once drainage of the necrotic collection has been indicated based on clinical, laboratory, and imaging data.
Finally, serum measurements of procalcitonin may be a valuable tool supporting the diagnosis of infected pancreatic necrosis in patients with acute necrotizing pancreatitis and without extrapancreatic infection [31,32].
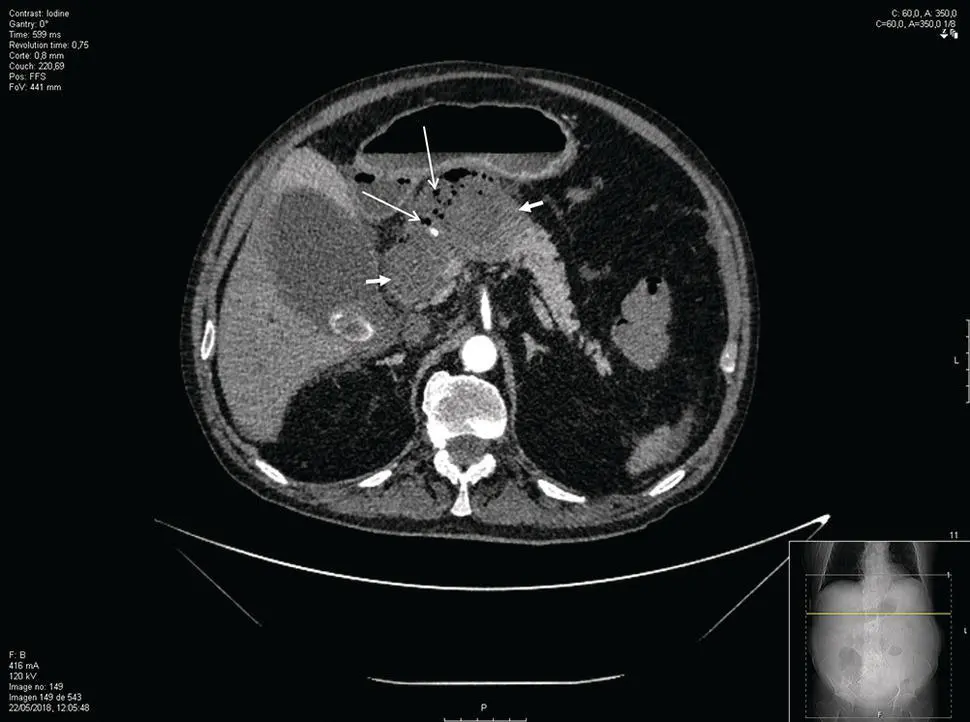
Figure 14.1 CT scan of a patient with walled‐off necrosis (short arrows). Presence of gas bubbles indicates the presence of infected (peri)pancreatic necrosis (long arrows).
Source: courtesy of J.E. Domínguez‐Muñoz.
How to Deal with Infected (Peri)pancreatic Necrosis
Infected pancreatic necrosis is associated with a high complication and mortality rate. Optimal management requires a multidisciplinary approach, including at least gastroenterologists, surgeons, interventional radiologists, and specialists in critical care medicine. Based on this approach, patients with severe disease should be transferred to high‐volume specialized centers.
Infected pancreatic necrosis is currently managed following a minimally invasive step‐up approach. This approach is based on the stepwise use of (i) systemic antibiotics, (ii) endoscopic or percutaneous drainage, and (iii) endoscopic or percutaneous minimally invasive necrosectomy.
Intravenous antibiotics, usually carbapenems, are the first step in the treatment of infected pancreatic necrosis. Antifungal agents are not consistently recommended. About 15–30% of patients respond well to this conservative approach and do not require any invasive intervention [33,34]. How long antibiotic therapy should be maintained is an open question that should be evaluated in each individual patient. If clinical and laboratory markers of sepsis do not improve after 48–72 hours of antibiotic therapy, infected WON should be drained.
Endoscopic or Percutaneous Drainage
Pancreatic drainage should be avoided in the early phase of the disease (first two weeks) and should be optimally delayed for four weeks when a mature wall has developed. Drainage of infected WON is effective in 35–70% of cases without the need for an additional necrosectomy [11,17].

Figure 14.2 CT scan of a patient with infected walled‐off necrosis after placement of a transgastric lumen apposing metal stent (short arrows) and a 7 Fr tube deep in the collection for local antibiotic infusion (long arrows).
Source: courtesy of J.E. Domínguez‐Muñoz.
Drainage of infected necrosis can be performed either endoscopically (endoscopic ultrasound guided) or percutaneously (CT guided). If drainage fails to treat infected necrosis, endoscopic drainage is followed by endoscopic necrosectomy, and percutaneous drainage is followed by laparoscopic necrosectomy according to the step‐up approach. Both approaches have been shown to be equally effective in terms of major complications or death, although the endoscopic approach is associated with less pancreatic fistulas and shorter hospital stay [17].
Based on a previously reported theoretical approach to local infusion of antibiotics for infected pancreatic necrosis [21], we have recently developed a protocol that combines local antibiotic therapy delivered via a single‐pigtail 7–8.5 Fr naso‐cystic tube with systemic antibiotics and endoscopic drainage for infected WON ( Figure 14.2). This method may reduce the need for necrosectomy by increasing the antibiotic concentration in the necrotic tissue. The efficacy of this approach should be tested in randomized controlled trials.
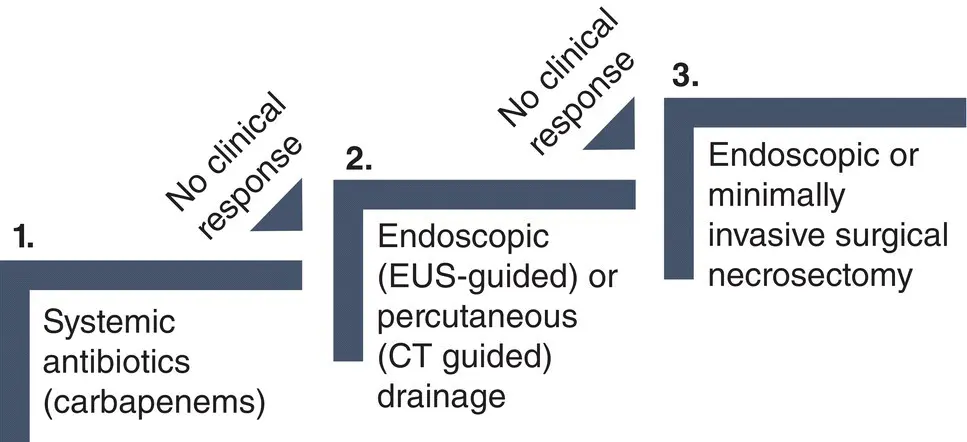
Figure 14.3 Step‐up approach for the management of infected pancreatic necrosis. EUS, endoscopic ultrasound.
Читать дальше















