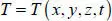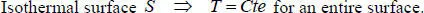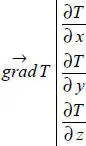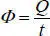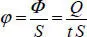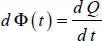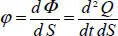And if this text has a moral, it would be: Writing down thermodynamics, just like thermal science, is based on continuous equations. The fundamentals of physics that determine these phenomena arise from the field of the discontinuous: discontinuity of matter, divided into particles; discontinuity of light, divided into photons .
1
The Problem of Thermal Conduction: General Comments
1.1. The fundamental problem of thermal conduction
The fundamental problem of thermal conduction involves the determination of temperature domains and flows across particular surfaces, for a given physical situation, in one or several given environments.
The resolution of a thermal conduction problem involves:
1 a) for all problems, a heat equation is considered, resulting from a local heat balance;
2 b) for specific conditions of the problem, the conditions are constituted at the limits that are applied to the heat equation.
In the most general case, these temperature domains T are not homogeneous. They can be three-dimensional and variable over time:
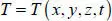
In other systems, we note that T = T ( r , θ , z , t ) or T = T ( r , θ , Φ, t ).
Temperature is expressed in degrees Celsius (°C), formerly degrees centigrade, or in Kelvin (K).
We know that the temperature, expressed in Kelvin, is measured from absolute zero. The conversion rule is known as: T K = T C + 273.15.
The most general problem presented in thermal conduction is therefore extremely complex, since the heat equation has four dimensions (three in space and one in time).
Fortunately, significant simplifications are possible for many problems.
The temperature field can only depend on a spatial variable. We say that conduction is monodimensional or bidimensional.
The temperature can remain fixed at each point as time progresses.
This last remark divides the approach that we will adopt into two parts:
– stationary conductive heat transfer;
– non-stationary conductive heat transfer.
Two important categories will be examined in this chapter:
– problems of stationary conduction with a single dimension: T = T(x) or T = T(r)
– problems of non-stationary conduction with a single dimension: T = T (x,t) or T =T (r,t)
For many problems, the analytical approach is possible. This will be the case, in particular, for the stationary or non-stationary problems with a single dimension. In (most) other cases, a numerical approach is required.
1.2. Definitions
1.2.1. Temperature, isothermal surface and gradient
The temperature T is a parameter defined in all thermodynamics classes.
As of now, we can define isothermal surfacesin space. An isothermal surface is a surface on which the temperature is constant:
[1.1] 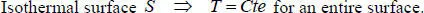
Furthermore, we will look at the important relationship between this surface and heat flow.
Let us note that in a non-stationary problem, the isothermal surface is mobile in time.
The temperature field also allows a very important vector to be defined, whose use will be explained later on: temperature gradient.
Initially, we define this vector in a Cartesian system. Later on, we will see that it is quite easy to determine its components in other systems when the symmetries in a problem make that a pertinent option.
In a Cartesian system, this gradient is constructed by taking, for each of its components, the partial derivative of the temperature with respect to the relevant coordinate axis. In other words:
[1.2] 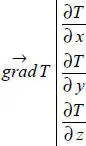
For other systems, it will be useful to refer to Appendix 2.
This mathematical operation is known to all physicists. With a minus sign, it is the derivation of a force from a potential.
We should note that in a stationary problem, this gradient is a vector attached to each point in space and fixed in time. In the case of a non-stationary problem, this gradient is a vector attached to each point in space and variable in time.
The temperature gradient possesses a fundamental property, which is demonstrated in Appendix 2: the gradient vector is normal at each point in space to the isothermal surface that passes through this point.
This property will be familiar to those who have studied electric fields.
1.2.2. Flow and density of flow
These two essential parameters will be used throughout this book.
Thermal flowΦ is the quantity of heat Q that passes a given surface in a unit of time:
[1.3] 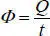
This flow is expressed in Joules per second (J.s -1) or even better, in Watts (W).
Density of the thermal flow φ , which can be even greater, is the flow that passes through a unit of surface:
[1.4] 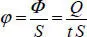
This flow density is expressed in Watt per m 2(W.m -2).
It is important not to confuse these two parameters, which also do not have the same units.
In this book, we will systematically note the flow with a capital Φ and the flow densities with a lowercase φ .
These flows and flow densities may not be uniform through a surface, which can also vary rapidly over time. We then deal with a definition on a differential basis, through small surface areas and with small time intervals.
Through a small surface d S :
[1.5] 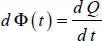
[1.6] 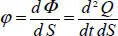
The quantity of heat d 2 Q is measured on a small surface d S and on a small time interval d t , which becomes twice differential. The flow d Φ ( t ) becomes differential since it is the relationship of a twice differential quantity by a small surface d S at a given time t . The flow density φ is the relationship between two differential quantities, which remains finite (expressed in flow per unit of surface and time).
1.3. Relation to thermodynamics
We have already pointed out that when we talk about thermal science, thermodynamics is not far off. More precisely, it is its main principle.
Before the first principle, there was calorimetry.
We should be aware that each time that we draw up a balance for an environment, which will be particularly true in Chapter 4, we link a variation in temperature to an exchange of heat by a calorimetric operation.
Calorimetry was, in fact, born before the first principle. We (mainly chemists) noted that the relative variations in temperature due to an exchange of what we had then designated as “heat”, depended on the mass m of the environments and a coefficient that is today denoted as the mass calorific capacity c . In other words, when a body transfers heat to another body, the relationship (with an obvious notation) is:
Читать дальше