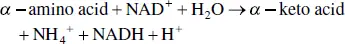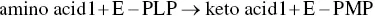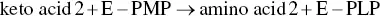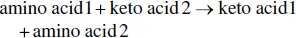6 The amino acids possessing an aromatic ring (tyrosine, phenylalanine, and tryptophan) are derived from erythrose 4‐phosphate and phosphoenolpyruvate. These two compounds are intermediates of the pentose cycle and glycolysis, respectively. Their condensation forms shikimate. The condensation of this compound with another molecule of phosphoenolpyruvate produces chorismate, aprecursor of aromatic amino acids.
2.4.2 Assimilation Mechanisms of Ammonium and Amino Acids
The penetration of ammonium and amino acids into the yeast cell activates numerous membrane protein transporters or permeases ( Section 1.3.2). Saccharomyces cerevisiae has at least two specific ammonium ion transporters (Dubois and Grenson, 1979). Their activity is inhibited by several amino acids, in a noncompetitive manner.
Two distinct categories of transporters ensure amino acid transport:
1 A general amino acid permease (GAP) transports all of the amino acids. The ammonium ion inhibits and represses GAP. GAP therefore appears to be active during winemaking only when the must no longer contains ammonium, i.e. after the end of the cell growth phase. It acts as a “nitrogen scavenger” toward amino acids (Cartwright et al., 1989).
2 Saccharomyces cerevisiae also has many specific GAPs (at least 11). Each one ensures the transport of one or more amino acids.
The transport of nitrogen sources is subject to complex regulations depending on the nitrogen content of the medium, by means of a system called nitrogen catabolite repression (NCR) (Beltran et al ., 2004).
Toward the end of fermentation, yeasts excrete significant but variable amounts of different amino acids. During fermentation, yeasts assimilate between 1 and 2 g/l of amino acids. Finally, at the end of alcoholic fermentation, a few hundred milligrams of amino acids per liter remain; proline generally represents half of this amount.
Contrary to must hexoses that penetrate the cell by facilitated diffusion, ammonium and amino acids require active transport. Their concentration in the cell is generally higher than in the external medium. The permeases involved couple the transport of an amino acid molecule (or ammonium ion) with the transport of a hydrogen ion. The hydrogen ion moves in the direction of the concentration gradient: the concentration of protons in the must is higher than in the cytoplasm. The amino acid and the proton are linked to the same transport protein and penetrate the cell simultaneously. This concerted transport of two substances in the same direction is called symport. Obviously, the proton that penetrates the cell must then be exported to avoid acidification of the cytoplasm. This movement is made against the concentration gradient and requires energy. The membrane ATPase ensures the excretion of the hydrogen ion across the plasma membrane, acting as a proton pump ( Figure 2.24).
Ethanol strongly limits amino acid transport. In an alcohol medium, it modifies the composition and the properties of the phospholipids of the plasma membrane. The membrane becomes more permeable to H +ions in the medium, and these ions massively penetrate the interior of the cell by simple diffusion. The membrane ATPase must increase its operation to control the intracellular pH. As soon as this task monopolizes the ATPase, the symport of the amino acids no longer functions. In other words, at the beginning of fermentation, and for as long as the ethanol concentration in the must is low, yeasts can rapidly assimilate amino acids and concentrate them in the vacuoles for later use, according to their biosynthesis needs.
2.4.3 Catabolism of Amino Acids
The ammonium ion is essential for the synthesis of amino acids necessary for building proteins, but yeasts cannot always find sufficient quantities in their environment. Fortunately, they can obtain ammonium from available amino acids through various reactions.
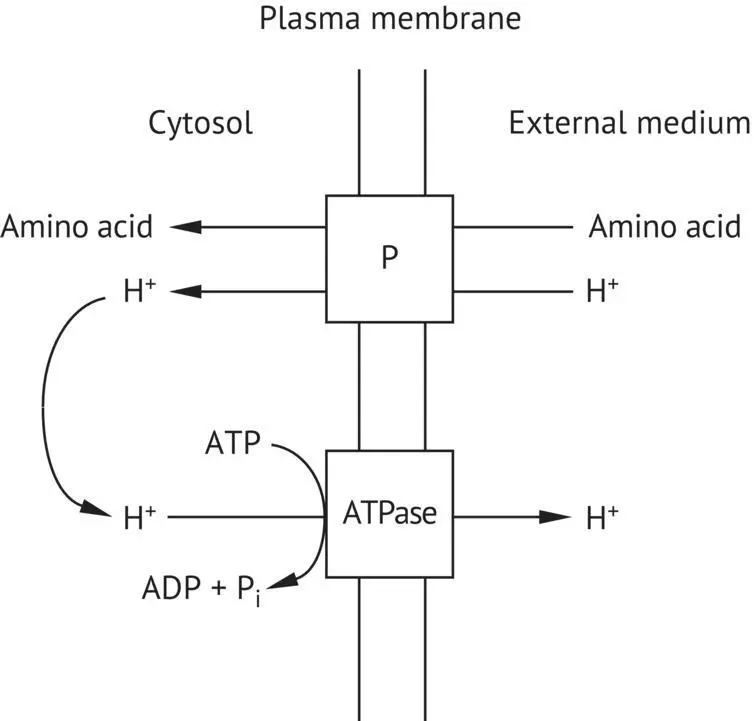
FIGURE 2.24 Active amino acid transfer mechanisms in the yeast plasma membrane. P, protein playing the role of an amino acid “symporter.”
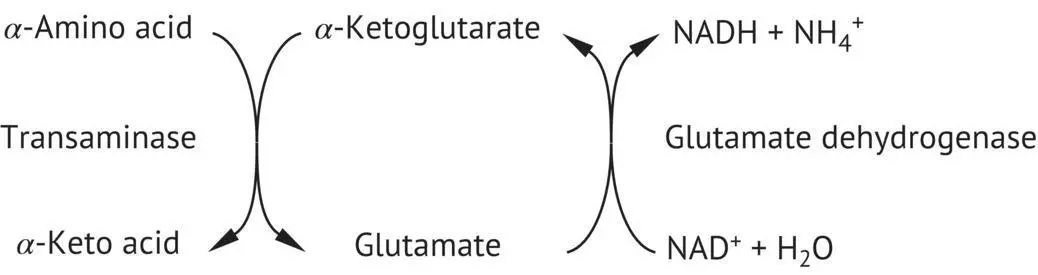
FIGURE 2.25 Oxidative deamination of an amino acid, catalyzed by a transaminase and glutamate dehydrogenase.
The most common pathway is the transfer of an α ‐amino group, originating from one of many different amino acids, onto α ‐ketoglutaric acid to form glutamate. Aminotransferases or transaminases catalyze this reaction, whose prosthetic group is PLP. Glutamate is then deaminated by an oxidative pathway to form NH 4 +( Figure 2.25). These two reactions can be summarized as follows:
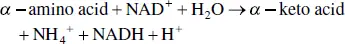
During transamination, PLP is temporarily transformed into pyridoxamine phosphate (PMP). The PLP aldehyde group is bound to a lysine residue ε ‐amino group on the active site of the aminotransferase to form an intermediate (E‐PLP) ( Figure 2.26). The α ‐amino group of the amino acid that is the transamination substrate displaces the lysine ε ‐amino group bound to PLP. The cleavage of this intermediate liberates PMP and the keto acid corresponding to the amino acid substrate. PMP can in turn react with another keto acid to furnish a second amino acid and regenerate PLP.
The partial reactions can be written in the following manner:
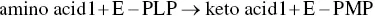
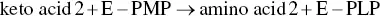
the balance for which is:
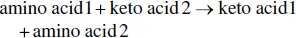
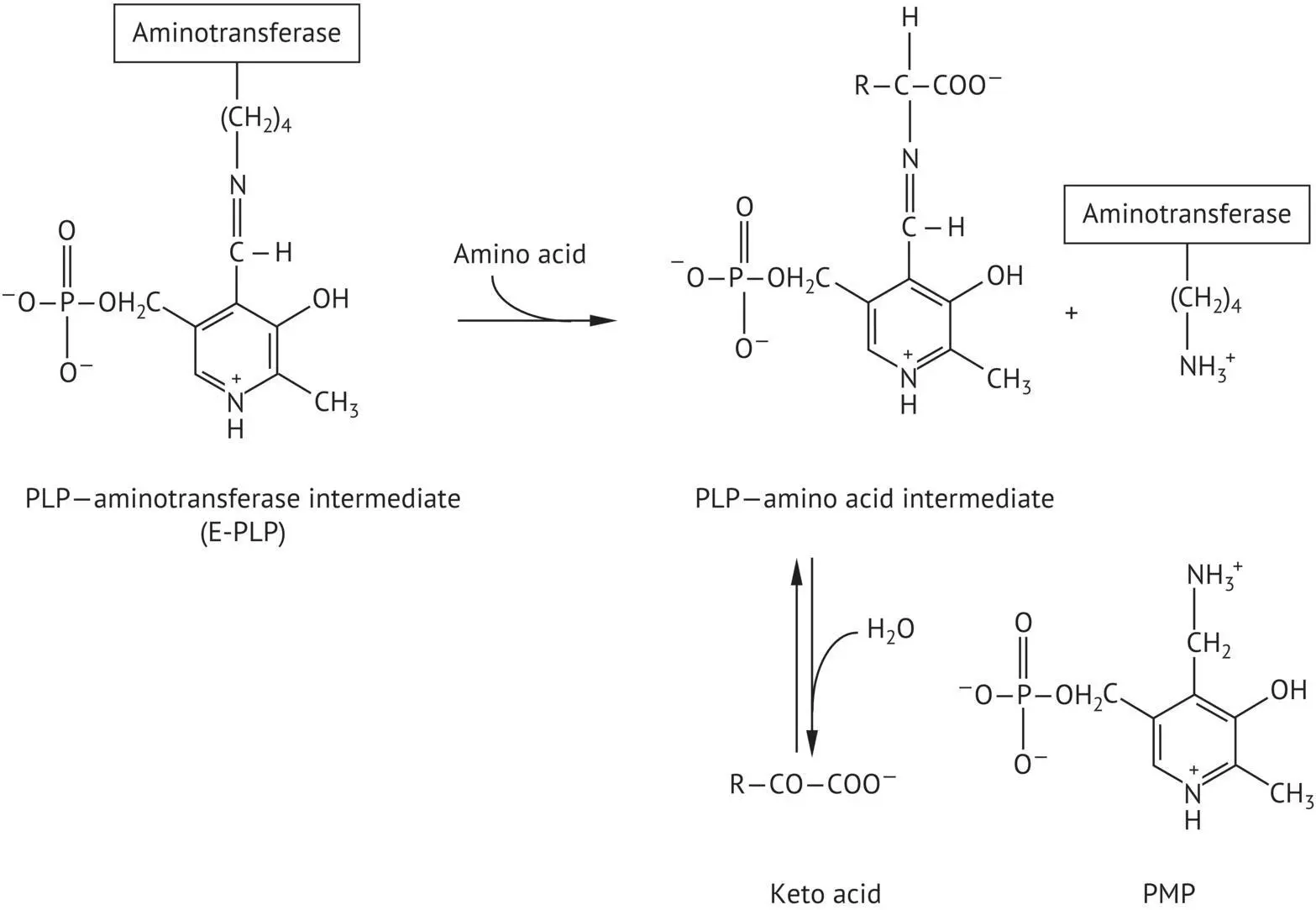
FIGURE 2.26 Mode of action of pyridoxal phosphate (PLP) in transamination reactions. Formation of intermediates between PLP and aminotransferase or the amino acid substrate.
Some amino acids, such as serine and threonine, possess a hydroxyl group on their β carbon. They can be directly deaminated by dehydration. A dehydratase catalyzes this reaction, producing the corresponding keto acid and ammonium ( Figure 2.27).
2.4.4 Formation of Higher Alcohols and Esters
Yeasts can excrete keto acids originating from the deamination of amino acids only after their decarboxylation into aldehyde and reduction into alcohol ( Figure 2.28). This mechanism, known as the Ehrlich reaction, explains in part the formation of higher alcohols in wine. Table 2.4lists the principal higher alcohols and their corresponding amino acids, possible precursors of these alcohols.
Several experiments clearly indicate, however, that the degradation of amino acids is not the only pathway for forming higher alcohols in wine. In fact, certain ones, such as propan‐1‐ol and butan‐1‐ol, do not have amino acid precursors. Moreover, certain mutants deficient in the synthesis of specific amino acids do not produce the corresponding higher alcohol, even if the amino acid is present in the culture medium. There is no relationship between the amount of amino acids in must and the amount of corresponding higher alcohols in wine.
Читать дальше