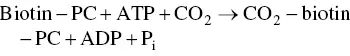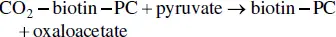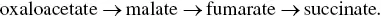Saccharomyces cerevisiae can metabolize ethanol via the respiratory pathway in the presence of small quantities of glucose. After alcoholic fermentation, oxidative yeasts develop in a similar manner on the surface of wine (Sections 14.5.2 and 14.5.3) as part of the process of making certain specialty wines (Sherry and vin jaune from Jura in France).
2.3.2 Regulation Between Alcoholic Fermentation and Glyceropyruvic Fermentation: Glycerol Accumulation
Wines contain about 8 g of glycerol per 100 g of ethanol. During grape must fermentation, about 8% of the sugar molecules undergo glyceropyruvic fermentation and 92% undergo alcoholic fermentation. The fermentation of the first 100 g of sugar forms the majority of the glycerol, after which glycerol production slows but is never nil. Glyceropyruvic fermentation is therefore more than just an inductive fermentation that regenerates NAD +when acetaldehyde, normally reduced into ethanol, is not yet present. Alcoholic fermentation and glyceropyruvic fermentation overlap slightly throughout the fermentation process.
Pyruvic acid is derived from glycolysis. When this molecule is not used by alcoholic fermentation, it participates in the formation of secondary products. In this case, a molecule of glycerol is formed by the reduction of dihydroxyacetone.
Glycerol production therefore equilibrates the yeast endocellular oxidation–reduction potential or NAD +/NADH balance. This “relief valve” eliminates surplus NADH, which appears at the end of the synthesis of amino acids, proteins, and the oxidations that generate secondary products.
Some winemakers place too much importance on the sensory role of glycerol. This compound has a sugary taste similar to glucose. In the presence of other constituents of wine, however, the sweetness of glycerol is practically imperceptible. For the majority of tasters, even well trained, the addition of 3–6 g of glycerol per liter to a red wine is not discernible. Therefore, the pursuit of winemaking conditions that are more conducive to glyceropyruvic fermentation has no enological interest. On the contrary, the winemaker should favor a pure alcoholic fermentation and should strive to minimize glyceropyruvic fermentation. The production of glycerol is accompanied by the formation of other secondary products, derived from pyruvic acid, whose increased presence (such as carbonyl function compounds and acetic acid) decreases wine quality.
2.3.3 Secondary Products Formed from Pyruvate by Glyceropyruvic Fermentation
When a molecule of glycerol is formed, a molecule of pyruvate is also formed. The latter cannot be transformed into ethanol following its decarboxylation into acetaldehyde. Under anaerobic conditions, oxaloacetate is the means of entry of pyruvate into the cytosolic citric acid cycle. Although the mitochondria are no longer functional, the enzymes of the citric acid cycle are present in the cytoplasm. Pyruvate carboxylase (PC) catalyzes the carboxylation of pyruvate into oxaloacetate. The prosthetic group of this enzyme is biotin; it serves as a CO 2transporter. The reaction makes use of an ATP molecule:
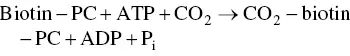
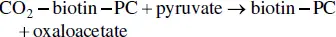
Under these anaerobic conditions, the citric acid cycle cannot be completed since the succinate dehydrogenase activity requires the presence of FAD, a strictly respiratory coenzyme. The chain of reactions is therefore interrupted at succinate, which accumulates ( Figure 2.7) up to levels of 0.5–1.5 g/l. The NADH generated by this portion of the citric acid cycle (from oxaloacetate to succinate) is reoxidized by the formation of glycerol from dihydroxyacetone.
Under anaerobic conditions, α ‐ketoglutarate dehydrogenase has a very low activity; some authors therefore believe that the oxidative reactions of the citric acid cycle are interrupted at α ‐ketoglutarate. In their opinion, a reductive pathway of the citric acid cycle forms succinic acid under anaerobic conditions:
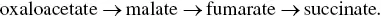
Bacteria have a similar mechanism. Camarasa et al . (2003) demonstrated that this is the main pathway found in S. cerevisiae yeast under anaerobic conditions. Furthermore, additional succinate is formed during alcoholic fermentation in a glutamate‐enriched medium. Glutamate is deaminated to form α ‐ketoglutarate, which is oxidized into succinate.
Among secondary products, compounds with a ketone function (pyruvic acid and α ‐ketoglutaric acid) and acetaldehyde predominantly bind with sulfur dioxide in wines made from healthy grapes. Their excretion is significant during the yeast proliferation phase and decreases toward the end of fermentation. Additional acetaldehyde is released in the presence of excessive quantities of sulfur dioxide in must. A high pH and high fermentation temperature, anaerobic conditions, and a deficiency in thiamine and pantothenic acid increase the production of ketoacids. Adding thiamine to must limits the accumulation of ketone compounds in wine ( Figure 2.10).
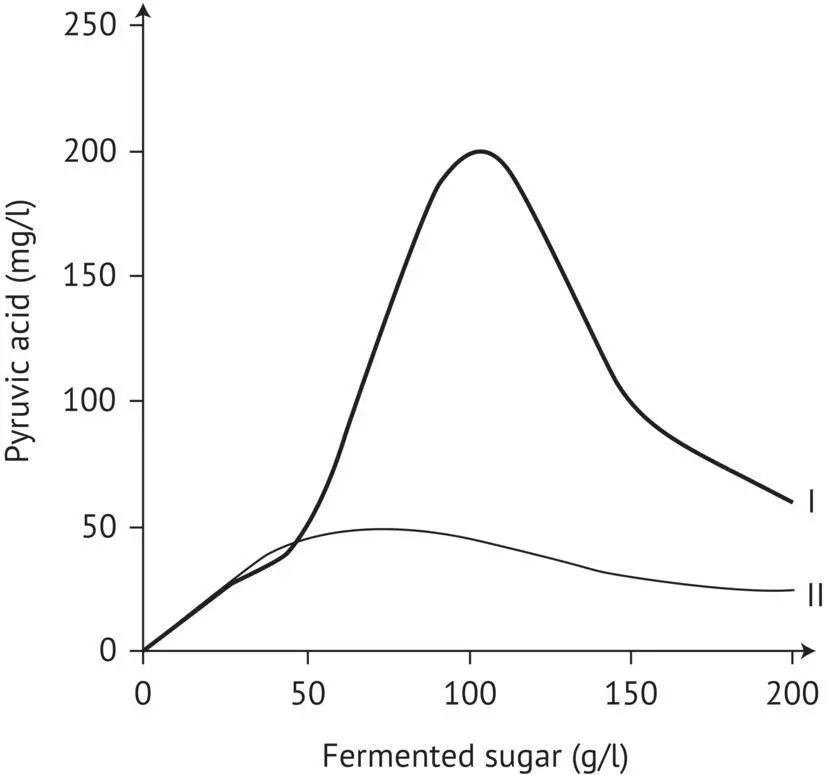
FIGURE 2.10 Effect of thiamine addition on pyruvic acid production during alcoholic fermentation (Lafon‐Lafourcade, 1983). I, control must; II, thiamine‐supplemented must.
Other secondary products of fermentation are also derived from pyruvic acid: acetic acid, lactic acid, butanediol, diacetyl, and acetoin. Their formation mechanisms are described in the following paragraphs.
2.3.4 Formation and Accumulation of Acetic Acid by Yeasts
Acetic acid is the principal volatile acid in wine. It is produced in particular during bacterial spoilage (acetic acid spoilage and lactic acid spoilage) but is always formed by yeasts during fermentation. Beyond a certain limit, which varies depending on the wine, acetic acid has a detrimental sensory effect on wine quality. In healthy grape must with a moderate sugar concentration (less than 220 g/l), S. cerevisiae produces relatively small quantities (100–300 mg/l), varying according to the strain. However, under certain winemaking conditions, even without bacterial contamination, yeast acetic acid production can be abnormally high and becomes a problem for the winemaker.
The biochemical pathway for the formation of acetic acid in wine yeasts has not yet been clearly identified. The hydrolysis of acetyl‐CoA can produce acetic acid. Pyruvate dehydrogenase produces acetyl‐CoA beforehand by the oxidative decarboxylation of pyruvic acid. This reaction takes place in the mitochondrial matrix but is limited under anaerobic conditions. Aldehyde dehydrogenase can also form acetic acid by the oxidation of acetaldehyde ( Figure 2.11). This enzyme, whose cofactor is NADP +, is active during alcoholic fermentation. The NADPH thus formed can be used to synthesize lipids. When pyruvate dehydrogenase is repressed, this pathway forms acetyl‐CoA through the action of acetyl‐CoA synthetase. Under anaerobic conditions in a model medium, yeast strains producing the least amount of acetic acid have the highest acetyl‐CoA synthetase activity (Verduyn et al ., 1990).
Читать дальше