Next, glycolysis ( Figure 2.2) is carried out entirely in the cytosol of the cell. It includes a first stage, in which glucose is converted into fructose 1,6‐bisphosphate, requiring two ATP molecules. This transformation itself comprises three steps: an initial phosphorylation of glucose into glucose 6‐phosphate, the isomerization of the latter into fructose 6‐phosphate, and a second phosphorylation forming fructose 1,6‐bisphosphate. These three reactions are catalyzed by hexokinase, phosphoglucoisomerase, and phosphofructokinase, respectively.
In fact, Saccharomyces cerevisiae has two hexokinases (PI and PII) capable of phosphorylating glucose and fructose. Hexokinase PII is essential and is active predominantly during the yeast log phase in a sugar‐rich medium. Hexokinase PI, partially repressed by glucose, is not active until the stationary phase (Bisson, 1993).
Mutant strains devoid of phosphoglucoisomerase have been isolated. Their inability to develop on glucose suggests that glycolysis is the only catabolic pathway of glucose in S. cerevisiae (Caubet et al ., 1988). The pentose phosphate pathway, by which some organisms utilize sugars, serves only as a means of synthesizing ribose 5‐phosphate, incorporated in nucleic acids and in reduced nicotinamide adenine dinucleotide phosphate (NADPH) in Saccharomyces .
The second stage of glycolysis forms glyceraldehyde 3‐phosphate. Under the catalytic action of aldolase, fructose 1,6‐bisphosphate is cleaved, thus forming two triose phosphate isomers: dihydroxyacetone phosphate and glyceraldehyde 3‐phosphate. Triose phosphate isomerase catalyzes the isomerization of these two compounds. Although at equilibrium, the ketose form is more abundant than the aldose form, the transformation of dihydroxyacetone phosphate into glyceraldehyde 3‐phosphate is rapid, since this compound is continually eliminated by the ensuing glycolysis reactions. In other words, a molecule of glucose leads to the formation of two molecules of glyceraldehyde 3‐phosphate.
The third phase of glycolysis is composed of two steps that recover part of the energy from glyceraldehyde 3‐phosphate. Initially, it is converted into 1,3‐bisphosphoglycerate (1,3‐BPG). This reaction is catalyzed by glyceraldehyde 3‐phosphate dehydrogenase. It is an oxidation coupled with a substrate‐level phosphorylation. Nicotinamide adenine dinucleotide (NAD +) is the cofactor of the dehydrogenation. At this stage, it is in its oxidized form; nicotinamide is the reactive part of the molecule ( Figure 2.3). Simultaneously, an energy‐rich phosphate ester bond is established between the oxidized carbon of the substrate and the inorganic phosphate. NAD +accepts two electrons and a hydrogen atom lost by the oxidized substrate. Next, phosphoglycerate kinase catalyzes the transfer of the phosphoryl group of the acylphosphate from 1,3‐BPG to ADP; and 3‐phosphoglycerate and ATP are formed.
The last phase of glycolysis transforms 3‐phosphoglycerate into pyruvate. Phosphoglyceromutase catalyzes the conversion of 3‐phosphoglycerate into 2‐phosphoglycerate. Enolase catalyzes the dehydration of the latter, forming phosphoenolpyruvate. This compound has a high phosphoryl group transfer potential. By phosphorylation of ADP, pyruvic acid and ATP are formed; pyruvate kinase catalyzes this reaction. In this manner, glycolysis creates four ATP molecules. Two are immediately used to activate a new hexose molecule and the net gain of glycolysis is therefore two ATP molecules per molecule of hexose metabolized. This stage marks the end of the common trunk of glycolysis, which differentiates between alcoholic fermentation, glyceropyruvic fermentation, and respiration.
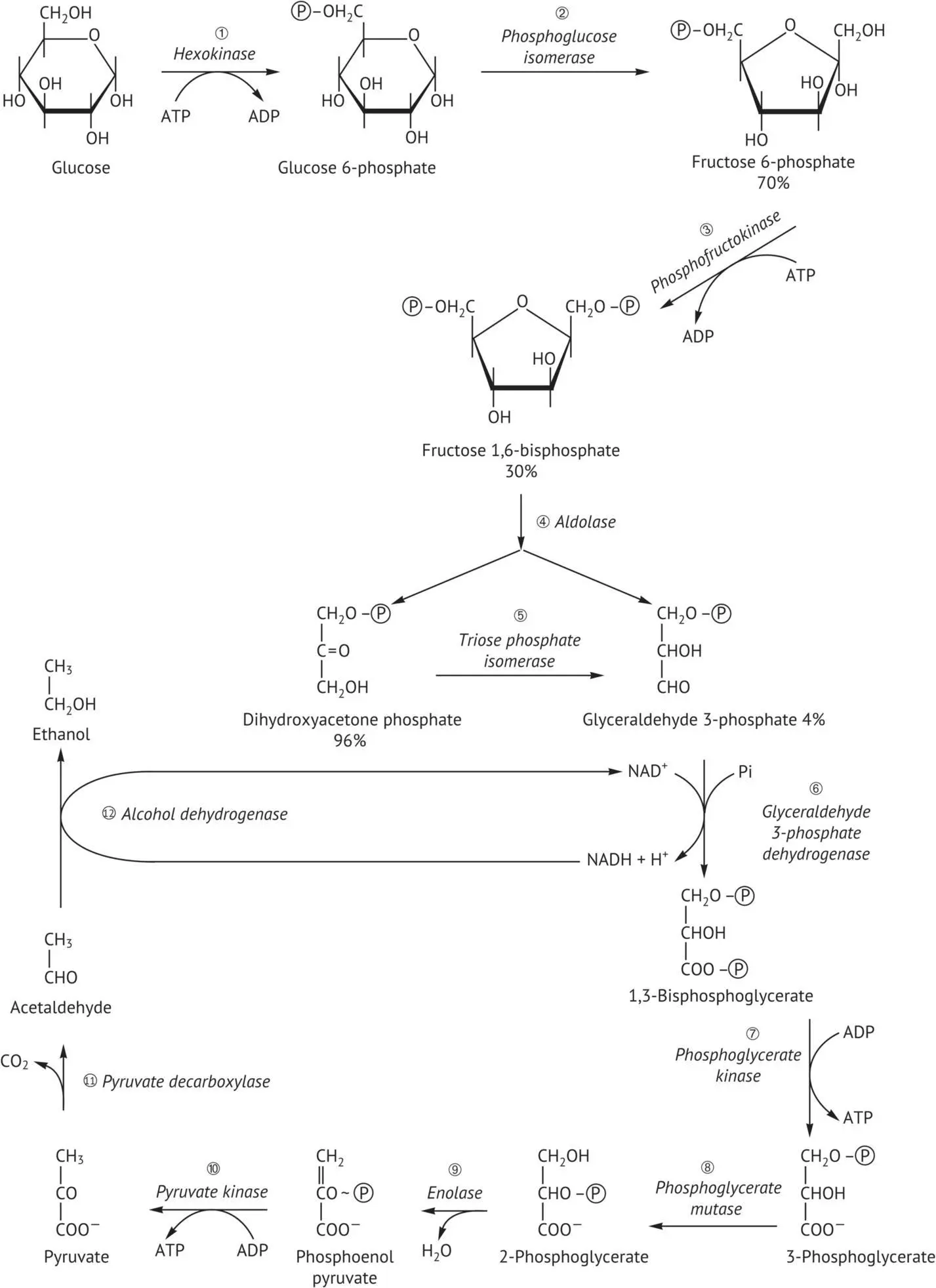
FIGURE 2.2 Glycolysis and alcoholic fermentation pathway.
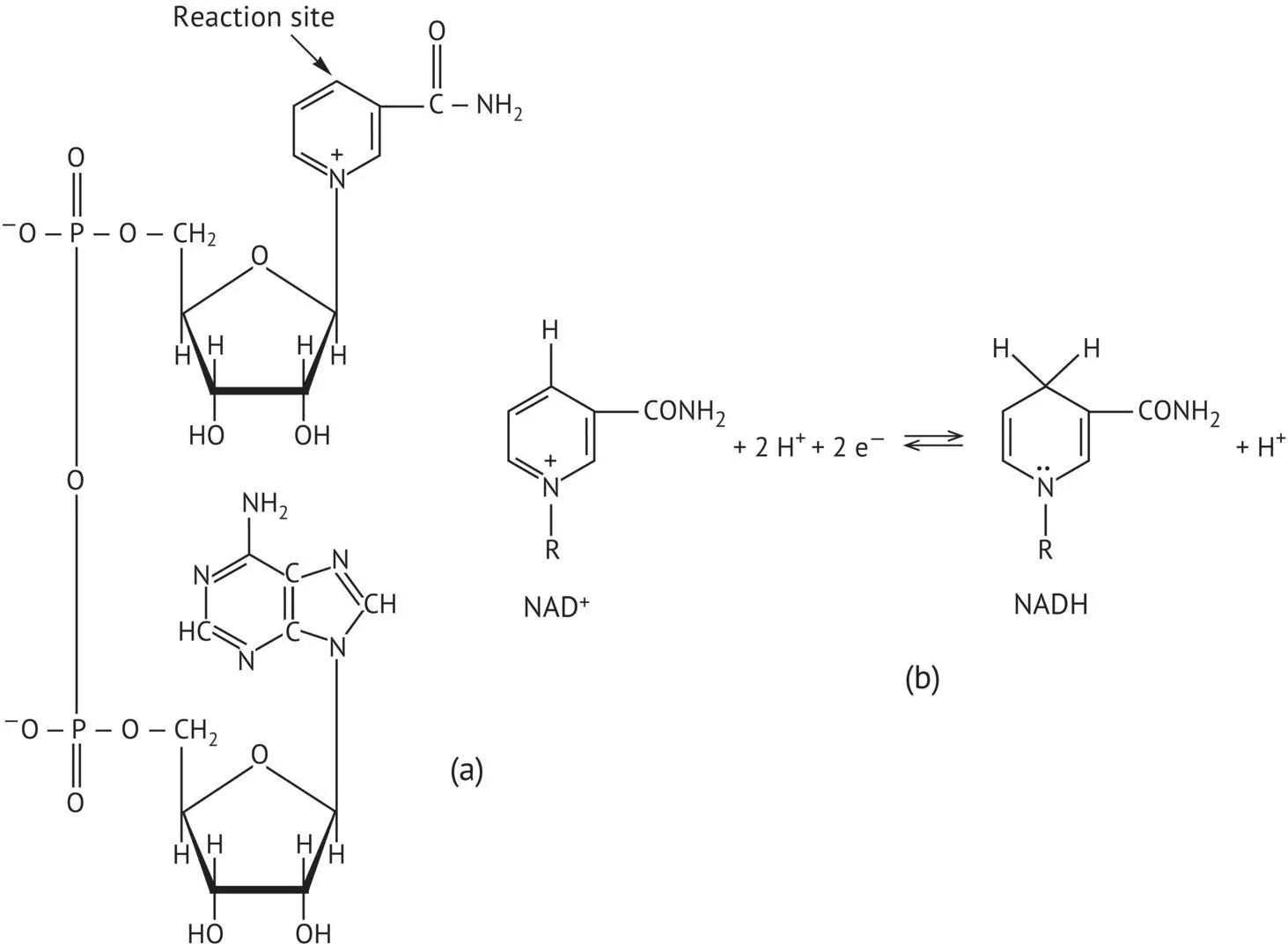
FIGURE 2.3 (a) Structure of nicotinamide adenine dinucleotide in the oxidized form (NAD +). (b) Equilibrium reaction between the oxidized (NAD +) and reduced (NADH) forms.
2.2.2 Alcoholic Fermentation
The reducing power of NADH produced by glycolysis must be transferred to an electron acceptor in order to regenerate NAD +. In alcoholic fermentation, it is not pyruvate but rather acetaldehyde, its decarboxylation product, that serves as the terminal electron acceptor. With respect to glycolysis, alcoholic fermentation contains two additional enzymatic reactions.
The first decarboxylates pyruvic acid, catalyzed by pyruvate decarboxylase (PDC). The cofactor is thiamine pyrophosphate (TPP) ( Figure 2.4). TPP and pyruvate form an intermediate compound. More precisely, the carbon atom located between the nitrogen and the sulfur of the thiazole ring of TPP is ionized. It forms a carbanion, which readily combines with the pyruvate carbonyl group. The second step reduces acetaldehyde into ethanol by NADH. This reaction is catalyzed by alcohol dehydrogenase, whose active site contains a Zn 2+ion.
Saccharomyces cerevisiae PDC comprises two isoenzymes: a major form, PDC1, representing 80% of the decarboxylase activity, and a minor form, PDC5, whose function remains uncertain.
From an energy viewpoint, glycolysis followed by alcoholic fermentation therefore supplies the yeast with two molecules of ATP per molecule of glucose degraded or 14.6 biologically usable kcal per mole of glucose fermented. From a thermodynamic viewpoint, the change in free energy during the degradation of a mole of glucose into ethanol and CO 2is −40 kcal. The difference (25.4 kcal) is dissipated in the form of heat.
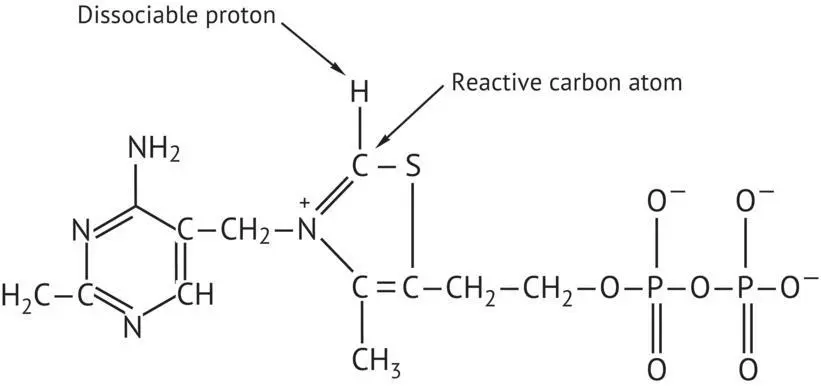
FIGURE 2.4 Structure of thiamine pyrophosphate (TPP).
2.2.3 Glyceropyruvic Fermentation
In the presence of sulfite (Neuberg, 1946), the fermentation of glucose by yeasts produces equivalent quantities of glycerol, carbon dioxide, and acetaldehyde in its bisulfite form. This glyceropyruvic fermentation takes place in the following manner. Since the sulfite‐bound acetaldehyde cannot be reduced into ethanol, dihydroxyacetone phosphate becomes the terminal acceptor of electrons from the oxidation of glyceraldehyde 3‐phosphate, which is then reduced to glycerol 3‐phosphate. The latter is dephosphorylated into glycerol. This mechanism was used for the industrial production of glycerol. In this fermentation, only two molecules of ATP are produced for every molecule of hexose degraded. ATP is required to activate the glucose in the first step of glycolysis ( Figure 2.5). Glyceropyruvicfermentation, whose net gain in ATP is nil, does not furnish biologically assimilable energy for yeasts.
Glyceropyruvic fermentation does not occur solely in a highly sulfited environment. At the beginning of alcoholic fermentation of grape must, the inoculum consists of yeasts initially grown in the presence of oxygen. Their PDC and alcohol dehydrogenase are weakly expressed. As a result, acetaldehyde accumulation is limited. The reoxidation of NADH therefore does not involve acetaldehyde, but rather dihydroxyacetone. Glycerol, pyruvate, and some secondary fermentation products are formed. These secondary products are pyruvate derivatives—including, but not limited to, succinate and diacetyl.
Читать дальше















