1.3.8 Resolution of inflammation in obesity and diabetes
The increase of pro-inflammatory mediators is a key factor in DM, and elevated circulating IL-1β, IL-6 and CRP are predictive of T2DM. In addition, adipose tissue is an endocrine organ, which regulates appetite, glucose and lipid metabolism as well as blood pressure, inflammation and immune function. It also operates as an insulating energy store. Consequently, the resolution of adipose tissue-derived inflammation would be expected to bring a beneficial therapeutic effect, reducing the risk of developing obesity-associated complications, such as insulin resistance and T2DM 204. In T2DM, uncontrolled inflammation has detrimental effects, and dysregulation of resolution is a possible link to the severity of the disease.
It is now recognised that the resolution of inflammation is a dynamically regulated process orchestrated by mediators that play important counter-regulatory roles including cytokines, chemokines and lipid mediators such as specialised pro-resolving mediators (SPMs). These are lipoxins, resolvins and protectins 205. SPMs actively reduce neutrophil infiltration, promote neutrophil apoptosis 206, and recruit nonphlogistic macrophages that clear apoptotic neutrophils and remaining bacteria 207. To complete the resolution phase, macrophages are themselves cleared by other macrophages, a process termed efferocytosis 208. Interestingly, the signalling pathways initially inducing pro-inflammatory mediator formation and thus the onset of inflammation, switch from the production of pro-inflammatory to pro-resolving lipid mediators by inducing 5-lipoxygenase that leads to the production of SPMs. In this way, physiological inflammation programmes its own resolution and promotes tissue homeostasis 209.
T2DM is associated with impaired phagocytosis and bacterial killing by cells of the innate immune system, leading to an increase in infection susceptibility. Neutrophils and monocytes are primed due to the chronic hyperglycaemia in poorly controlled T2DM subjects, resulting in an exaggerated inflammatory response and tissue damage 210 , 211. SPM are produced via the actions of specific lipoxygenases on different substrates, including arachidonic acid-derived lipoxins (LXA4 and LXB4), eicosapentaenoic acid (EPA)-derived E-series resolvins (RvE1–3), docosahexaenoic acid (DHA)-derived D-series resolvins (RvD1–6), maresins and protectins 212. Resolvins, such as resolvin E1 (RvE1), are potent agents against inflammation-related diseases such as asthma 213, retinopathy 214and periodontitis 215 - 217. They are biosynthesised from the omega-3 fatty acids EPA and DHA.
Genetically engineered animals have been used to study the impact of T2DM on the inflammatory response. Herrera et al 218demonstrated that the exogenous administration of RvE1 increased phagocytosis, killing, and clearance of the periodontal pathogen P. gingivalis in healthy (wild type [WT]) mice but not in T2DM model mice, which are genetically obese leptin receptor-deficient animals. However, in mice with an overexpression of the RvE1 receptor, ERV1, P. gingivalis phagocytosis and killing were significantly greater in response to RvE1 in vitro in both normoglycaemic and diabetic mice 218. Another study, by Freire et al 219, demonstrated that ERV1 is responsive to inflammatory stimuli (lipopolysaccharides, TNF-α and leukotriene B4) and that the lipid mediator ligand RvE1 improves inflammatory responses, such as P. gingivalis phagocytosis, in human neutrophils from subjects with uncontrolled T2DM.
Taken together, uncontrolled inflammation present in T2DM subjects could be the key factor for its association with other diseases, such as cardiovascular and periodontal diseases. So far, the traditional focus when managing T2DM has been the control of hyperglycaemia and insulin, not the resolution of inflammation. In the future, mediators that promote the resolution of inflammation should be considered as potential therapeutic targets, for both DM and periodontitis.
SUMMARY
● Microbial factors: studies demonstrated the bacterial shift only in people with poorly controlled diabetes, but there is insufficient evidence of a causal relationship between poorly controlled DM and periodontal microbial dysbiosis.
● Cytokines and adipokines: there is sufficient evidence for elevated levels of IL-1β, IL-6 and RANKL/OPG ratios in patients with diabetes and periodontitis as compared to patients with periodontitis alone.
● Immune cell function: there is limited evidence from experimental studies for a role of altered monocyte, T cell and aberrant neutrophil function in people with diabetes and periodontitis.
● Hyperglycaemia, AGEs and RAGE: diabetes drives the irreversible formation of AGEs that have direct pro-inflammatory and oxidative damage effects on cells and thus the periodontal tissues.
● Hyperglycaemia and alveolar bone homeostasis: changes in the RANKL/OPG axis and an inflammatory state in diabetes play an important role in alveolar bone loss during periodontitis in individuals with diabetes.
● There is evidence from clinical and experimental studies to support the role of specific cytokines, such as CRP, TNF-α and IL-6, in the relationship between diabetes and periodontitis. Furthermore, in patients with periodontitis, diabetes is associated with elevated levels of several pro-inflammatory cytokines and other mediators in saliva and GCF.
● Pro-resolving lipid mediators are a promising and potential novel target in the therapy of DM and periodontitis, due to their ability to terminate inflammation and promote healing.
Whether the existing findings represent truly causal interrelationships remains to be determined. The strength of evidence to suggest a bidirectional biological relationship between DM and periodontitis is summarised in Fig 1-6.
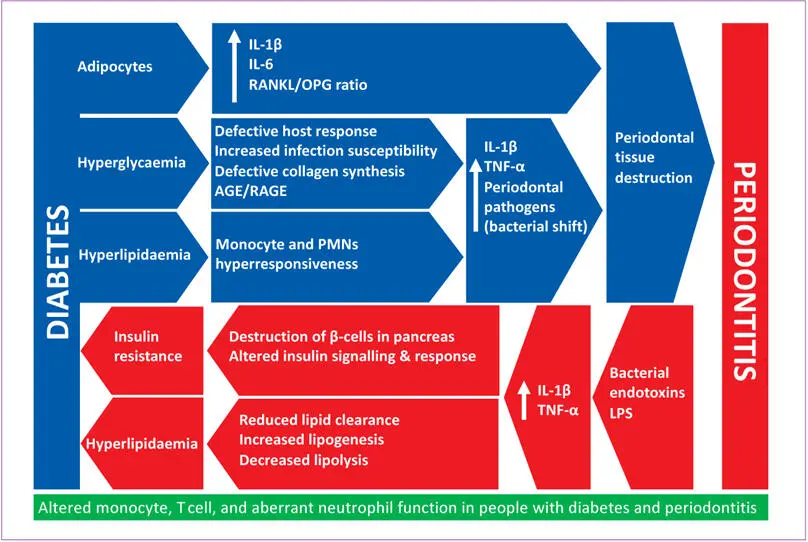
Fig 1-6 Biological rationale for the bidirectional interrelationship between diabetes and periodontitis. Adapted from Grover and Luthra 220. (AGE = advanced glycation end product; IL = interleukin; LPS = lipopolysaccharide; OPG = osteoprotegerin; PMN, polymorphonuclear neutrophil; RAGE = cell surface receptors for AGE; RANKL = receptor activator of nuclear factor kappa B ligand; TNF-α = tumour necrosis factor alpha.)
1.4 Guidelines for prevention and treatment of patients with diabetes
The International Diabetes Federation and the European Federation of Periodontology has published consensus guidelines for physicians, oral health care professionals and patients 221. These guidelines also apply to people with pre-diabetes and metabolic syndrome. In summary, they state that oral health education should be provided to all patients with diabetes. Patients with diabetes should be informed that periodontitis risk is increased, and if untreated, that periodontitis has a negative impact on metabolic control and may also increase the risk of complications of their diabetes such as cardiovascular and kidney disease. Therefore, successful periodontal therapy may have a positive impact upon their metabolic control and can prevent diabetes complications.
Physicians should investigate a prior diagnosis of periodontitis. For all patients, disease testing should begin at the age of 45 years. If results are within the normal range, the test should be repeated at 3-year intervals, but in those with pre-diabetes, the re-test and risk status should be assessed annually. In case of a positive diagnosis, the physician should ascertain whether periodontal care and maintenance are being provided. If patients are symptomatic (polydipsia, polyuria, polyphagia, unexplained weight loss), they should be referred directly to a physician. Nasseh et al 222found a statistically significant association between periodontal intervention and lower health care costs. However, this association was only noted among individuals who did not initiate diabetes drug therapy after diagnosis. For instance, individuals newly diagnosed with T2DM, who had undergone periodontal intervention, had total health care costs that were $1799 lower on average over years 3 and 4 compared with those who did not have periodontal therapy. These data suggest not only a positive effect of periodontal therapy on the course of DM, but also on the economic burden imposed by DM.
Читать дальше












![John Bruce - The Lettsomian Lectures on Diseases and Disorders of the Heart and Arteries in Middle and Advanced Life [1900-1901]](/books/749387/john-bruce-the-lettsomian-lectures-on-diseases-and-disorders-of-the-heart-and-arteries-in-middle-and-advanced-life-1900-1901-thumb.webp)
