In 1992, Matsuoka et al. reported a oligo( p ‐phenylenes) organic molecular photocatalyst for CO 2photoreduction to formic acid with small quantity of CO in a nonaqueous solvent under UV light illumination [52]. To further improve the selectivity of formic acid and efficiency, Tamaki and coworkers designed a series of Ru(II) supermolecular photocatalysts that exhibit high electivity and turnover frequency (TOF) under visible‐light irradiation [53]. By means of combining photosensitizer units and catalyst units, a trinuclear complex displayed 0.061 of formic acid yield, 671 of TON, and 11.6 min −1of TOF. Recently, Tamaki et al. prepared a novel sacrificial electron donor, 1,3‐dimethyl‐2‐( o ‐hydroxyphenyl)‐2,3‐dihydro‐1 H ‐benzoimidazole, for efficient supermolecular photocatalyst, further increasing reduction efficiency of CO 2to HCOOH, where the TON HCOOHand TOF HCOOHreached to 2766 and 44.9 min −1, respectively, under visible‐light irradiation [54].
Owing to the high cost and unrecyclable properties of homogeneous photocatalysts, chemical scientists pay more attention to combine solid semiconductor photocatalysts with liquid organic supermolecular photocatalysts together to overcome these obstacles as well as maintain high efficiency and selectivity. For instance, Suzuki et al. reported that a hybrid photocatalyst that consisted of Ru(II) complex and N‐doped Ta 2O 5anchored with an organic group was fabricated through a direct assembly route [55]. In Figure 2.11a, when the N‐Ta 2O 5was excited by suitable light source, the photogenerated electrons at the CB of N‐Ta 2O 5transferred to the Ru complex, in which the two‐electron reduction of CO 2to HCOOH can be easily triggered. Nevertheless, the solid semiconductor and organics should be assembled by a certain organic ligand to realize electron transfer process. To reach the goal, phosphonate and carboxylic groups were introduced to link the inorganic and organic components. The inorganic/organic system modified by phosphonate or carboxylic groups exhibited excellent photoconversion activity of CO 2to formic acid under visible‐light irradiation with respect to the reaction rate and stability, as shown in Figure 2.11b. On the other hand, the rapid and efficient electron transfer can be achieved by adjusting the position of CB of semiconductor. For example, recently, Maeda and coworkers developed a Ru(II) complex/C 3N 4hybrid photocatalyst for improving the photocatalytic reduction efficiency of CO 2to HCOOH (selectivity >98%) under longer wavelength visible ( λ > 500 nm) through a copolymerization preparation method, as shown in Figure 2.11c–d [56]. For the copolymerized C 3N 4nanosheets fabricated by combing urea with phenylurea in high temperature, the absorption edge can be extended to 650 nm, which can significantly enhance photocatalytic activity and selectivity of modified Ru(II) complex/C 3N 4system to HCOOH under longer‐wavelength light source irradiation.
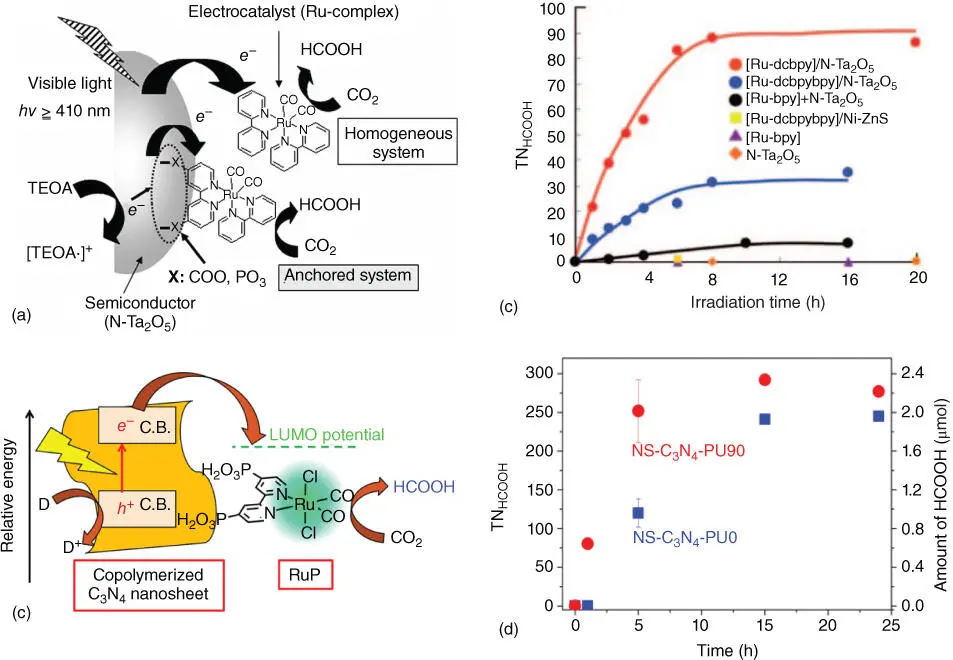
Figure 2.11(a) Mechanism for the reduction of CO 2by photocatalysis under visible light with a Ru complex and an N‐Ta 2O 5hybrid catalyst. (b) Turnover number for HCOOH formation from CO 2over various molecular systems as a function of irradiation time. (c) Schematic mechanism of visible‐light‐driven photocatalytic CO 2reduction over RuP/NS‐C 3N 4hybrids. (d) Time courses of photocatalytic CO 2reduction using RuP/NS‐C 3N 4‐PU0 and RuP/NS‐C 3N 4‐PU90 under visible light ( λ > 400 nm).
Source: (a) Suzuki et al. [55]; (b) Sato et al. [55]; (c) Tsounis et al. [56]; (d) Tsounis et al. [56].
From the reaction procedure of CO 2photoreduction, the reductive electrons mainly react with the adsorbed carbon species into C1 products because these reactions can be more easily driven thermodynamically. On the other hand, more valuable hydrocarbons including ethane and ethanol are more desirable such that it is inevitable to proceed multistep reactions involving more complicated reaction mechanism. It is well known that a multistep reaction means decaying of reaction efficiency and increasing of by‐products. Therefore, selectivity is a key parameter for the production of C2 or higher hydrocarbons.
In general, there are two possible ways of CO 2reduction to C 2H 6, which is from hydrogenation of ethylene intermediate or dimerization of two CH 3adsorbates. Recently, Choi and his group introduced a thin Nafion layer on Pd‐deposited TiO 2nanoparticles that markedly enhances the photosynthetic conversion of CO 2to hydrocarbons (mainly CH 4and C 2H 6) in an aqueous suspension without any sacrificial electron donor under UV and solar irradiation conditions [57]. In this system, the Nafion layer functioned as a conductive and fixed layer to facilitate diffusion of protons onto the CO 2reduction sites and immobilize intermediates of CO 2reduction, which could help the serial electron transfers from the intermediate to the final product. The hybridized catalyst displays the CH 4and C 2H 6production of 123 and 12 μmol g cat −1, which apparently inhibited H 2generation. Besides, Kulandaivalu et al. constructed a CQDs/Cu 2O nanocomposite photocatalyst with good optical, physical, and chemical properties that was able to selectively reduce CO 2to C 2H 6under visible‐light irradiation [58]. The improved photoactivity is the accumulated C 2H 6of 101.83 μmol g cat −1for six hours under continuous visible‐light illumination, while the pure Cu 2O photocatalyst produces C 2H 6of 66.73 μmol g cat −1for the same reaction time. The findings present that no methane or ethylene is detected over bare Cu 2O and CQDs/Cu 2O hybrids, indicating that the dimerization of CH 3adsorbates is favorable in this system. The proposed step number of C 2H 6production is eight, where both the photoinduced electrons and holes take part in the CO 2photoreduction and 3.5 molecules of O 2for each C 2H 6molecular is released from water splitting process but no hydrogen is detected.
Apart from ethane, ethanol is also reported in the photoreduction of CO 2in the past [59]. Recently, Liu et al. reported visible‐light‐responsive photocatalyst BiVO 4for selective formation of ethanol under the condition of high‐intensity visible‐light irradiation [60]. It is known that the proton in water cannot capture the photogenerated electrons on BiVO 4to produce H 2. In contrast, once CO 2molecule is adsorbed and changed to CO 3 2−, the photogenerated electrons can react with CO 3 2−likely and give rise to methanol after protonation. Furthermore, under intense irradiation, a large number of C1 intermediate species are anchored on the surface of BiVO 4, which will facilitate dimerization of C1 intermediate species to form ethanol. Based on this strategy, Peng and coworkers took advantage of graphitic carbon nitride with high CB position for achieving selectively photocatalytic reduction of CO 2under visible‐light irradiation [61]. It was found that the starting materials of graphitic C 3N 4have significant influence on the activity and selectivity of CO 2reduction. Once graphitic carbon nitride was fabricated by using urea (denoted as u‐ g ‐C 3N 4) that possessed large surface area with mesoporous nanostructure, the reduced products of CO 2were a mixture of CH 3OH and C 2H 5OH in the presence of NaOH (1.0 M), where the yields were 6.28 and 4.51 μmol g cat −1h −1, respectively. Compared with u‐ g ‐C 3N 4, the sample prepared by using melamine (denoted as m‐ g ‐C 3N 4) mainly catalyzed CO 2to C 2H 5OH under the same conditions, where the yield of C 2H 5OH was 3.64 μmol g cat −1h −1. Meanwhile, high concentration O 2and trace H 2were detected in two samples, inferring that photoexcited holes at VB of C 3N 4can be efficiently formed from water splitting and the corresponding electrons took part in the conversion of CO 2with H +into alcohols. Moreover, by comparing u‐ g ‐C 3N 4with m‐ g ‐C 3N 4, it shows that larger surface area, smaller crystal size, and lower crystallinity may be the main reasons for the apparent difference in selective formation of CH 3OH and C 2H 5OH in the present system. Owing to the nonporous structure of m‐ g ‐C 3N 4, the fast exchange of the formed ·OCH 3or CH 3OH may be suppressed, thereby in turn benefiting the dimerization to produce C 2H 5OH in the present CO 2/NaOH system.
Читать дальше



![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)









