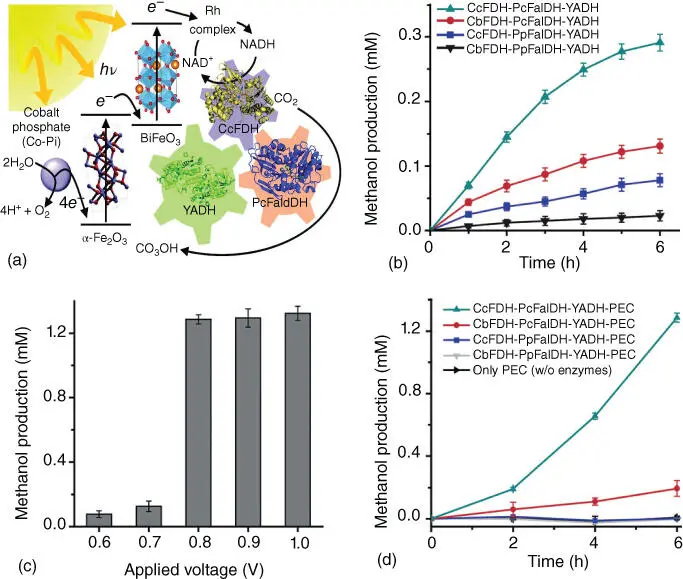
Figure 2.10(a) Schematic illustration of the solar‐assisted production of methanol from CO 2and water through a three‐enzyme cascade. (b) Methanol production as a function of time in multienzymatic systems. (c) Photoelectrochemical production of methanol by TPIEC under various applied potentials under visible‐light illumination. (d) Methanol production of the TPIEC system in various multienzymatic systems with an external voltage of 0.8 V.
Source: Kuk et al. [41].
2.4.1.3 Formaldehyde (HCHO)
As described in Eq. (2.3), HCHO is one of products that can be theoretically and experimentally formed, involving four‐electron reactions, while more elementary reactions happen during the final formation of HCHO [42]. Meanwhile, HCHO is also detected as an intermediate for fulfilling next reactions and producing CO and other advanced hydrocarbons. For example, Ojha et al. found that HCHO and COO– functioned as important intermediates to form CO over Cu‐modified S‐doped g ‐C 3N 4thin sheets through in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) [43]. Meanwhile, it is found that the existing forms of Cu in S‐doped g ‐C 3N 4system include Cu 0, Cu +, and Cu 2+three states, which have obvious effects in the adsorbed species and reaction pathways. Owing to the higher absorbance value on the Cu 0‐decorated sheets, the bicarbonate species as an intermediate tended to promote CH 4production. Furthermore, Song and coworkers explore the selectivity and photocatalytic activity of Cu‐ or Ni‐doped TiO 2catalysts by means of a hydrolysis method for the reduction of CO 2under irradiation from Hg lamps in a CO 2/NaOH aqueous solution [44]. The results display that CO, HCHO, CH 3OH, and CH 4can be yielded simultaneously in Cu/TiO 2and Ni/TiO 2system, while methane is identified as the major product. At the optimum value of 1%, the CO 2conversion ability and light utilization ability reach to 29.43 μmol g −1and 244.72 μmol g cat −1, respectively, which is almost seven times that of pure TiO 2after 24 hours UV illumination. In Ni‐doped TiO 2, the maximum value of the CO 2conversion ability and light utilization ability, amounting to 43.97 and 460.30 μmol g cat −1, respectively, was observed after 24 hours UV irradiation. The CO 2conversion ability can be indirectly defined as the sum of the mole number of all independent carbon products, including CH 3OH, CO, HCHO, and CH 4. And meanwhile, the transformation of the photon is used to evaluate the light utilization ability, which is determined not only by the total quantity of carbon products produced but also by the H 2yield. Therefore, the light utilization ability is the sum of the CO 2conversion ability and the H 2yield, which indicates that H 2is the main product instead of reduced hydrocarbons. Recently, Garay‐Rodríguez et al. reported a Ba 3Li 2Ti 8O 20/CuO composite for selective reduction of CO 2to formaldehyde under visible‐light irradiation [45]. The highest formaldehyde production is 11.6 μmol g cat −1h −1, which is mainly attributed to lower particle size distribution and higher surface area compared with others, allowing an increase in the CO 2adsorption.
On the other hand, owing to the ability to uptake and release electron, polyoxometalate (POM) can prolong the lifetime of the intermediate by directing the flow of the charge carriers, which enhances the efficiency of photocatalytic activity in CO 2reduction [46]. For example, Barman et al. prepared a Mo‐based polyoxometalate for selective formation of formaldehyde in photochemical carbon dioxide reduction [47]. In this system, a maximum of 0.858 mmol of formaldehyde and 0.63 mmol of formic acid were obtained in the reactions with 0.01 μmol of catalyst loading. The overall maximum turnover number (TON) for photochemical carbon dioxide reduction is 546. Meanwhile, the results present that water as an electron donor releases O 2, electrons, and protons simultaneously through water oxidation process that is a pH‐dependent reaction. A plausible mechanism of HCOOH formation is proposed, where CO 2attached on Mo‐based polyoxometalate is converted to CO 2anion radical under the attack of the electron and subsequently is reduced to HCOO– and formic acid in the presence of large protons. Finally, formic acid is detached to vacate the active site to further produce formaldehyde.
To further improve the selectivity of HCHO in CO 2photoreduction, the reaction parameters and reactors have been studied in the past, as similar as other chemical reactions. Recently, Brunetti et al. reported a continuous operating mode using C 3N 4–TiO 2photocatalyst embedded in a dense Nafion matrix, where reaction pressure as a driving force was specifically and systematically investigated to determine reactor performance and product removal in photocatalytic CO 2reduction [48]. The results showed that under the high feed pressure (5 bar), HCHO is the predominant production and MeOH is the secondary, where the carbon conversion efficiency achieved is 61 μmol g cat −1h −1, better performing than other photocatalytic membrane reactors. Meanwhile, the effects of H 2O/CO 2feed molar ratio and contact time on the membrane reactor performance were explored. Total converted carbon instead did not vary significantly with reaction pressure.
2.4.1.4 Formic Acid (HCOOH)
Formic acid, the two‐electron reduction product of CO 2, has recently attracted attention as a storage source of H 2. Formic acid itself is an important chemical. It has been employed as a preservative and an insecticide and is also a useful acid, reducing agent, and source of carbon in synthetic chemical industries. Analyzing the reduction reaction of CO 2to HCOOH, as shown in Eqs. ( 2.8, 2.10), it is found that this reaction involves multiple steps and multiple electrons so that the efficiency and yield are relatively low and the by‐products undermine the selectivity of HCOOH through a series of competitive reactions. Therefore, unique photocatalysts or photoelectrocatalysts should be developed to meet the need of CO 2conversion to HCOOH:
(2.8) 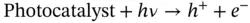
(2.9) 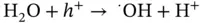
(2.10) 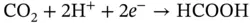
Recently, bimetallic‐graphene (Pt@Au@rGO) system has been reported by Kim and coworkers, in which the hybridized system is composed of gold nanoparticles (30 nm) as the core and ultrasmall platinum nanoparticles as the satellites wrapped by RGO [49]. The results demonstrate that the noble metal nanoparticles can efficiently improve the selectivity for HCOOH formation because noble metal can decrease the overpotential of hydrogen production from water splitting, therefore beneficial to the hydrogenation of CO 2, in particular Pt nanoparticles compared with other noble metals. On the other hand, it is reported that CB of g ‐C 3N 4has enough high position, located at −1.23 V, which is more negative than the reduction potential of CO 2/CH 3OH (−0.38 V) and CO 2/HCOOH (−0.61 V). Therefore, it is often viewed as an effective photocatalyst for CO 2photoreduction to methanol and formic acid. For example, Adekoya et al. constructed g ‐C 3N 4/(Cu/TiO 2) nanocomposites through coupling g ‐C 3N 4with Cu‐modified TiO 2to suppress the rapid recombination of photoinduced charge carriers in pristine g ‐C 3N 4[50]. As a result, the nanocomposite photocatalyst displayed enhanced photoreduction abilities of CO 2to CH 3OH and HCOOH under UV and visible‐light irradiation. Meanwhile, the yield of HCOOH (5069 μmol g cat −1) is much higher than that of CH 3OH (2574 μmol g cat −1), but the selectivity is not good. According to the analysis of HCOOH formation and related literatures, it seems that higher CB potential and lower overpotential hydrogen facilitate the yield of HCOOH, while it is observed that the by‐product CH 3OH is higher, which is a strong competitive reaction due to the lower redox potential compared with that of HCOOH synthetic reaction [51]. Consequently, only introducing new component for enhanced charge separation and solar harvesting is hardly to achieve high selectivity of HCOOH. Therefore, novel photocatalysts with new reaction path of HCOOH should be considered and developed.
Читать дальше


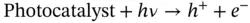
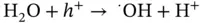
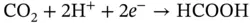

![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)









