Many of the issues introduced here will also be discussed in greater detail in later chapters.
This section of the protocol is intended to give a concise overview of the aims of the clinical trial. The precise structure will depend on the features of the protocol that need to be highlighted. Of particular importance will be the type of subjects concerned, the interventions planned and the chosen design. This may take a structured format, as one might have when submitting the trial eventually for publication, or may be a bulleted list that is perhaps accompanied with a schema, such as that of Figure 2.1, summarising the design.
Example 3.1 Protocol SQGL02 (1999): Brimonidine as a neuroprotective agent in acute angle‐closure glaucoma (AACG)
This is an open‐labelled, randomized, active‐controlled prospective pilot study comparing the efficacy of Brimonidine 0.2% with Timolol 0.5% as a neuroprotective agent in preventing/reducing visual field defects in patients with acute angle‐closure glaucoma (AACG). 80 patients presenting with AACG at 4 centes (SGH/SNEC, TTSH, NUH & CGH) will be recruited into the study and randomized to Timolol or Brimonidine, in addition to the standard medical treatment and laser peripheral iridotomy (PI) for AACG. Baseline Humphrey visual fields program 24–2 will be performed after PI. The study medication will be continued for 1 month and the visual fields compared at the end of 4 months to determine if either group has better preservation of fields. If Brimonidine is found to be efficacious in preserving visual fields and hence offering neuroprotection, a larger prospective randomized clinical trial may be planned subsequently to follow.
This statement was followed by a sentence describing the trial objectives, very brief eligibility requirements of ‘unilateral attack of AACG and informed consent’ only, and finally, a schema illustrating key aspects of the design to be implemented. The extract very clearly summarises the main features of the planned trial.
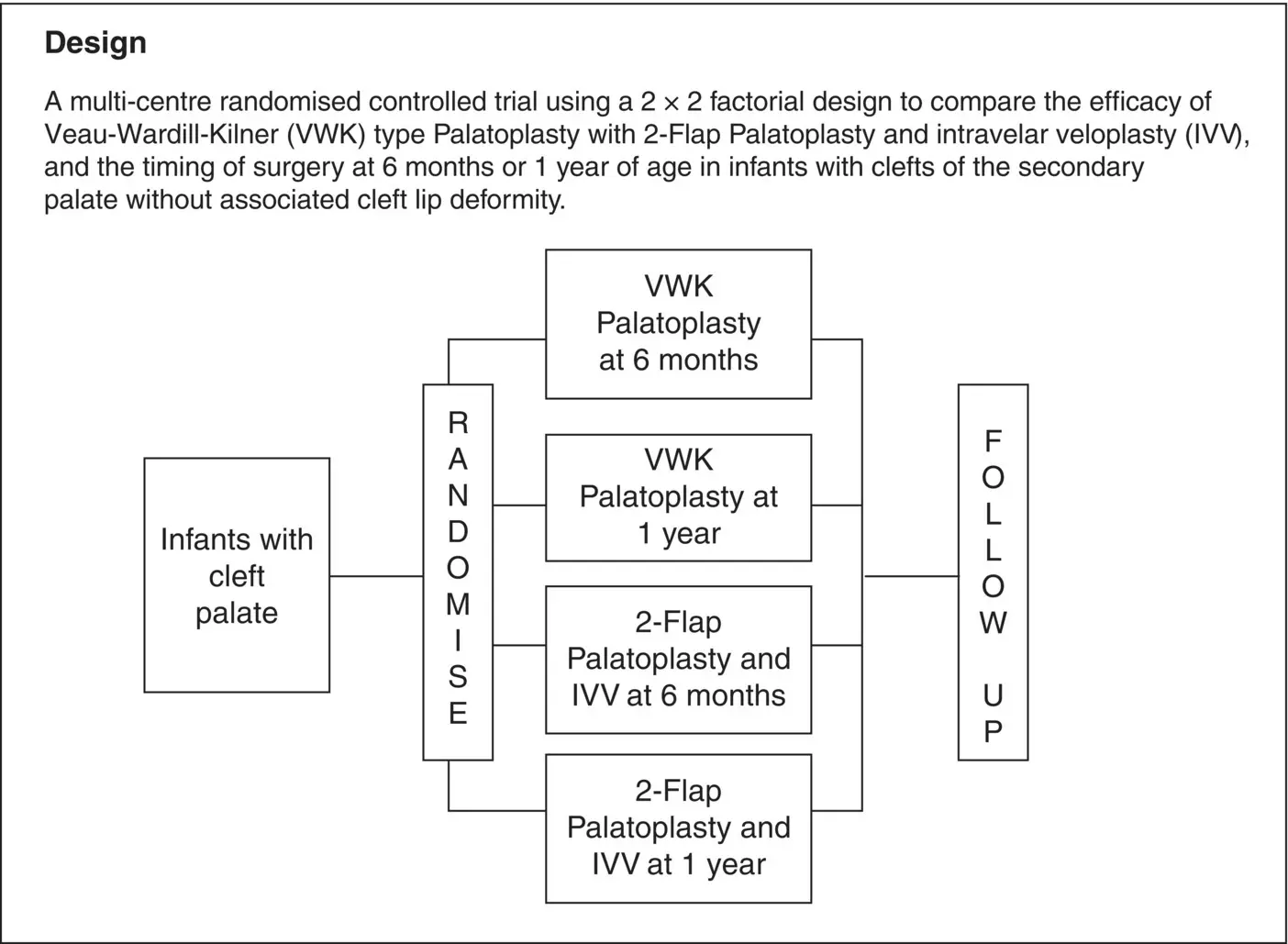
An abstract within a scientific journal would not normally contain a schema of the trial design due to space limitations. However, within a trial protocol, this may well be very helpful as an overview of the trial. Thus SQCP01 (2006) trial of Example 3.2below has an initial Summary page (as opposed to designating this as an Abstract) containing a structured review of Objectives, Eligibility, Method, and Design including an outline schema of their factorial trial. A more detailed schema is given in a later section of their protocol.
Example 3.2Protocol SQCP01 (2006): Comparing speech and growth outcomes between two different techniques and two different timings of surgery in the management of clefts of the secondary palate
Any individual or group concerned with answering an important clinical question by means of a clinical trial should be conversant with the medical speciality concerned and is likely to be expert within that discipline. Nevertheless, those planning a trial not only need to ensure they have the relevant team assembled but should still be prepared to seek outside assistance as appropriate. Thus, even at the early stages of formulating the research question, discussions with peers from relevant disciplines will always be valuable. Alongside this process, detailed reviews of the medical and related literature are required. These reviews can help formulate the research question itself, provide details on, for example, the safety of the interventions planned, and information on many other aspects of the intended trial. Once the question(s) is defined, a literature search may establish whether or not other trials have been conducted with the same or similar objectives and if so whether these either obviate the need for the trial in question or lead to some modification in its design.
The object of the background section within the trial protocol is to provide an in‐depth summary of how the proposed trial arose, with references to relevant published work, as well as the consequences for clinical practice and/or research once the trial results are known. Essentially this section would contain the information necessary for the Introduction that will be needed for the future research publication describing the trial results. Although it is difficult to be precise about the content the aim is to give an informed reader a clear rationale for justifying the importance and relevance of the clinical trial to be conducted. Thus, it must be persuasive enough to convince those who will be part of the formal approving process, for example, the local ethics committees of the participating clinical centres. It must also convince interested colleagues who may wish to participate. Thus, it should use language which is neither too specialised, nor cluttered with unnecessary detail, yet it must provide a clear scientific/clinical motivation for the randomised trial outlined.
In this section of the protocol, the research objectives of the trial are summarised in broad outline and specifics with regard to the endpoints provided.
The objectives need not be lengthy provided they encapsulate the major intentions – essentially stating the hypotheses under test. The examples given below do not refer explicitly to the design chosen, for example whether randomised or not, but do imply they are comparative in nature.
Example 3.3 Protocol SQNP01 (1997): Standard radiotherapy versus concurrent chemo‐radiotherapy followed by adjuvant chemotherapy for locally advanced (non‐metastatic) nasopharyngeal cancer
To compare the clinical response, distant metastases, disease free survival and overall survival of chemo‐radiotherapy and adjuvant chemotherapy using combination chemotherapy comprising CisDDP and 5‐Fluorouracil (5‐FU), with radiotherapy in patients with locally advanced NPC.
This summary indicates the comparisons concerned, although omitting full details of the (complex) chemotherapy regimen under test, and makes it clear the patient are those with a particular form of nasopharyngeal cancer. The statement also identifies the several trial endpoints to be determined, which concern response (and the protocol includes details of how this is to be assessed), measures that require following the patients progress to establish when (if ever) distant metastases or recurrent disease appear, and survival.
Example 3.4 Protocol PRESSURE (2000) Part Section 2: Pressure‐relieving support surfaces: a randomised evaluation
2. RESEARCH OBJECTIVES
2.1 Primary objective
To determine whether there are differences between alternating pressure overlays and alternating pressure replacement mattresses with respect to:
1 The development of new pressure sores
2 Healing of existing pressure sores
3 Patient acceptability of the surfaces
4 The cost‐effectiveness of the different pressure‐relieving surfaces.
To investigate the specific additional impact of pressure sores on patients’ wellbeing.
The research objectives of the PRESSURE (2000) trial clearly distinguish between the primary, although it is multiple in nature, and secondary objectives. They also specify the two mattress types, overlay and replacement, that are to be compared.
The eventual design chosen will have been influenced by the outcome measures, certainly by the primary one, and each endpoint measure must be explicitly defined. The trial structure in terms of, for example, the frequency and timing of the (possibly repeated) assessments should enable the endpoint for each individual subject on the trial to be determined. Thus, in the PRESSURE (2000) trial, in those patients admitted without pressure sores skin conditions were assessed daily by the ward nursing staff using the skin classification scale stipulated within the protocol.
Читать дальше