The larger molecules are disintegrated into smaller units by a destructive process, and these units are transformed into useful NPs, for example, grinding/milling, CVDs, physical vapour deposition, etc. [29]. This approach is generally used to synthesize NPs from coconut shells (CSs). The milling method is used, whereby raw CS powders were finely milled using ceramic balls and a well‐known planetary mill at different time intervals. Via other characterization techniques, the influence of the milling period on the overall size of the NPs is shown.
Furthermore, as time increases, the size of the NP crystallite (Scherrer equation) decreases. In this process, it was also found that the brownish colour faded away with the increment of each hour because of the reduced size of the NPs [30]. Various characterization techniques demonstrated the effect of milling time on the overall size of the NPs. The synthesis of spherical magnetite NPs from natural iron oxide (Fe 2O 3) ore was shown in the presence of organic oleic acid by a destructive top‐down method with a particle size ranging from 20 to 50 nm [31]. To synthesize spherical particles of colloidal carbon using a control scale, a primary top‐down route was used. The synthesis technique was based on the continuous chemical adsorption of polyoxometalates on the carbon interfacial surface. Adsorption has transformed black carbon aggregates into relatively smaller spherical particles with a high dispersion capacity and a narrow distribution of size [32]. Microstructures have found that the quantity of carbon particles is lower during the sonication period. Combined grinding and top‐down sonication techniques synthesized a sequence of transition metal dichalcogenide nanodots (TMD‐NDs) from their crystallites. Every TMD‐ND with a size of less than 10 nm shows excellent dispersion and is demonstrated by the narrow distribution of the measure [33]. Highly photoactive Co 3O 4NPs have recently been produced by top‐down laser fragmentation, i.e. a top‐down process with an average size of 5.8 ± 1.1 nm. Powerful laser irradiations produce well‐uniformed NPs with adequate oxygen vacancy [34].
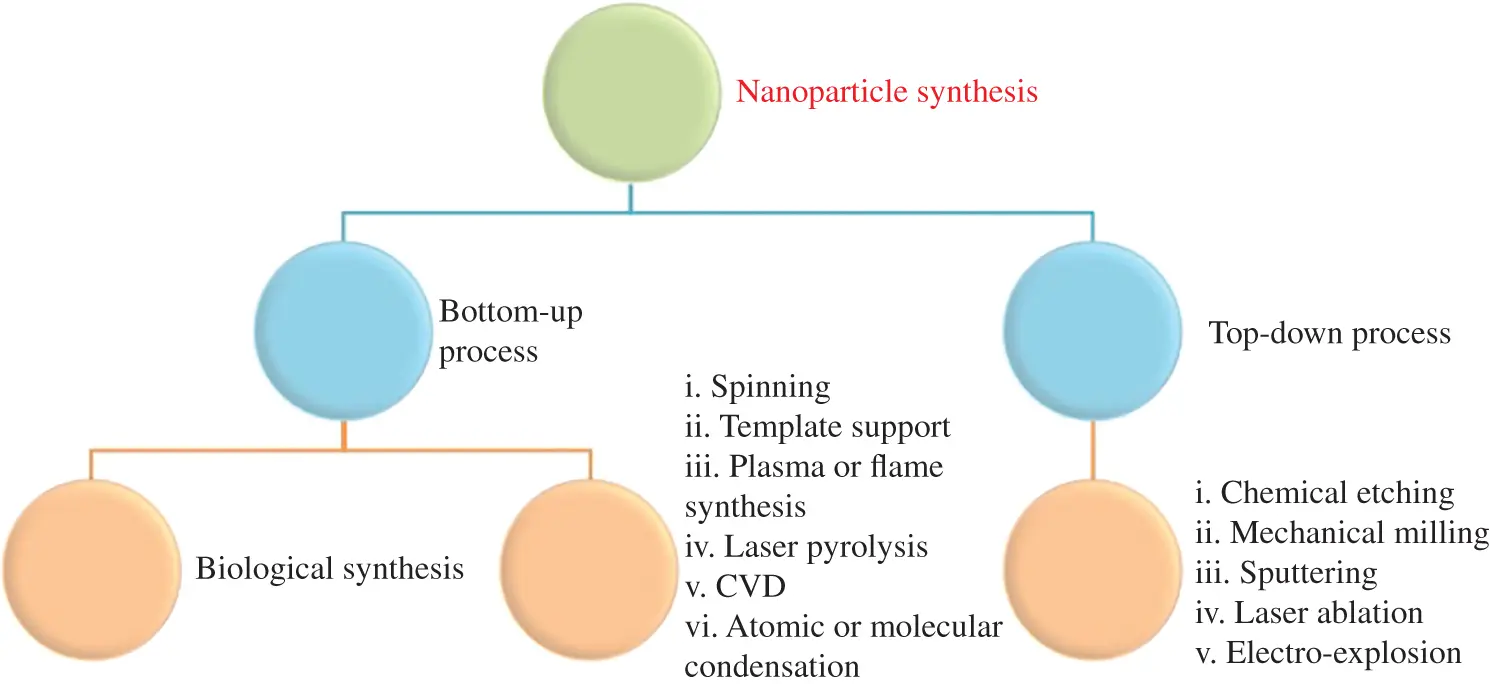
Figure 1.2 The synthetic models of NPs: top‐down and bottom‐up approach.
Source: Modified from Iravani [29].
1.3.2 Bottom‐Up Synthesis
This reverse approach is used to synthesize NPs from relatively more straightforward substances and is also called an approach to building up. Examples include sedimentation and reduction techniques. It encompasses sun‐freezing, green synthesis, spinning, and biochemical synthesis [29]. Mogilevsky et al. [35] used this technique to synthesize TiO 2anatase NPs to the graphene domains. Alizarin and titanium isopropoxide precursors have been used to synthesize the photoactive composite for methylene blue photocatalytic degradation. The X‐ray diffraction (XRD) framework has verified the anatase form [35]. Well‐uniform spherical shaped Au nanospheres have been synthesized with monocrystalline using top‐down laser irradiation technique [36, 37]. Recently, the solvent exchange method has been used to deliver medical cancer drugs to achieve limited‐size low‐density lipoprotein (LDL) NPs. The standard approach followed by growth, i.e. up process, is nucleation in this technique. The LDL NPs were obtained without phospholipid use and had high hydrophobicity, which is a prerequisite for drug delivery implementation. [38]. The monodispersed spherical bismuth (Bi) NPs, with top‐down and bottom‐up approaches, are synthesized with excellent colloidal properties [39]. In bottom‐up ethylene glycol, bismuth acetate was melted, although bismuth was converted into a molten state in the top‐down process. In boiled diethylene glycol, the molten drop then has been emulsified for NPs. Both methods generated different NPs in size from 100 to 500 nm [39] ( Figure 1.3a,b). Green and biogenic bottom‐up processing is cost‐effective and environmentally sustainable, where biological processes, such as using plant extracts, achieve the synthesis of NPs. For the synthesis of NPs, bacteria, yeast, fungi, Aloe vera , tamarind, and even human cells are used. Au‐NPs were synthesized from wheat biomass and oat [40] and used as a reduction agent by microorganisms and plant extracts [41, 42]. Figure 1.3demonstrated the bottom‐up ( Figure 1.3a) method: decomposing a molecular precursor into simple metal atoms, which transform into colloids and the top‐down ( Figure 1.3b) method.
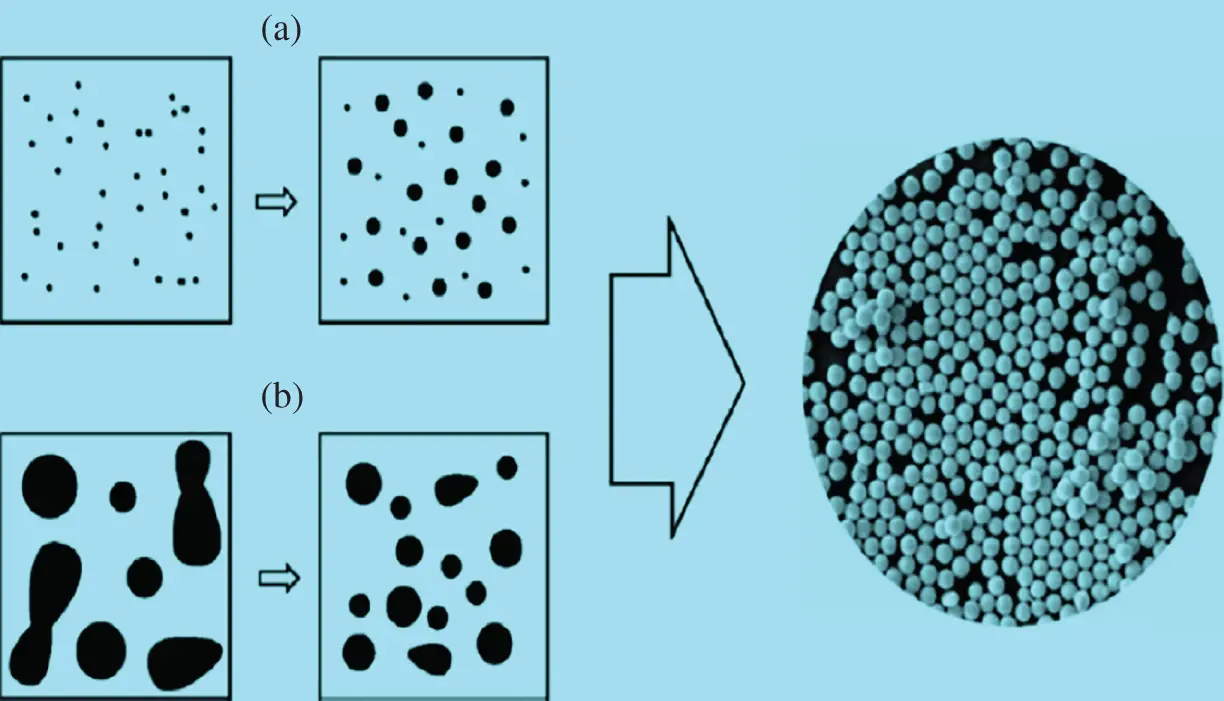
Figure 1.3 Illustration of synthesis of nanoparticles: (a) top‐down method and (b) bottom‐up method.
Source: From Wang and Xia [39]. © 2004, American Chemical Society.
1.4 NPs and Characterization
For the analysis of other physicochemical properties of NPs, different methods of characterization have been used, such as XRD, X‐ray photoelectron spectroscopy (XPS), infrared (IR), SEM, transmission electron microscopy (TEM), and Brunauer–Emmett–Teller (BET). Advanced methods are applied for the analysis of the particles.
1.4.1 Morphological Characterization
The morphological characteristics of NPs are still of great importance as morphology still affects most of the NP properties. Various characterization techniques exist for morphological research, but microscopic methods exist, such as polarized optical microscopy (POM), SEM, and TEM.
The SEM technique is based on the electron scanning principle and provides all the nanoscale NP data available. This technique is used to study the morphology of their nanomaterials and the dispersion of NPs in the bulk or matrix. This technique [15] showed the distribution of SWNTs in polymer matrix poly(butylene) terephthalate (PBT) and nylon‐6. The morphological characteristics of ZnO‐modified metal–organic frameworks (MOFs) were studied using the SEM technique, which indicates the dispersion of ZnO‐NPs and MOFs' morphologies under different reaction conditions [43].
It is based on the electron transfer principle, so that it can provide descriptions of the bulk content from very low to greater magnification. In addition, it is commonly used for the analysis of different morphologies of the Au‐NPs [44]. TEM also provides essential information about two‐ or more layer materials; the quadruple hollow shell structure of Co 3O 4NPs is observed by TEM, for instance. In Li‐ion batteries such as the anode, these NPs have proven themselves to be exceptionally efficient. The porous multi‐shell structure induces shorter Li +diffusion path lengths with ample annulled space for buffer volume expansion, good cycling efficiency, higher speed capacity, and essential capacity [45].
1.4.2 Structural Characteristics
The structural characteristics of the structure and function of the bonding materials are of primary importance for studying. It gives details about the bulk properties of the subject material. XRD, energy‐dispersive X‐ray (EDX) spectroscopy, XPS, IR, Raman spectroscopy, BET, and Zieta size analyser are the techniques used to study the structural properties of NPs.
One of the essential characterization techniques is to reveal the structural properties of NPs. The crystalline phase of NPs is provided with sufficient data. It also provides a rough image of the particle size through the Debye–Scherrer [8] formula. In the identification of single and multiphase NP [46] schemes, this approach worked well. However, in smaller NPs with a size smaller than hundreds of atoms, the acquisition and accurate measurement of structural and other parameters may be difficult. Besides, the XRD diffractogram can be affected by NPs with different interatomic lengths having more amorphous characteristics. To obtain accurate data, the diffractograms of bimetallic NPs must be contrasted with those of the corresponding monometallic NPs and their physical mixtures in this case. The best way to make a substantial difference is to measure the simulated bimetallic NP structural model with the spectra of XRD [47] observed.
Читать дальше













![Chade-Meng Tan - Search Inside Yourself - Increase Productivity, Creativity and Happiness [ePub edition]](/books/703803/chade-thumb.webp)
