Table 4.2brings together wavelengths of absorption maxima as well as molar extinction coefficients and fluorescence lifetimes and quantum yields of chlorophylls and bacteriochlorophylls in organic solvents. A comprehensive database of spectra and photophysical properties of chlorophylls and related pigments is available (Taniguchi and Lindsey, 2021). The spectral properties of these pigments are significantly altered when they are incorporated into protein complexes. In every case, the longest‐wavelength maximum shifts to longer wavelengths in pigment–proteins; sometimes the shift is more than 100 nm. We will examine the properties of these pigment–protein complexes in more detail in Chapters 5– 7.
Table 4.2 Spectroscopic properties of chlorophylls and bacteriochlorophylls in vitro a
Source: Data from Scheer (1991) and Niedzwiedzki and Blankenship (2010).
| Pigment |
λ max(nm) |
ɛ max(mM −1cm −1) b |
τ f(ns) |
φ f |
| Chlorophyll a |
662, 578,430 |
90.0 |
6.3 |
0.35 |
| Chlorophyll b |
644, 549, 455 |
56.2 |
3.2 |
0.15 |
| Chlorophyll c 1 |
640, 593, 462 |
35.0 |
6.3 |
|
| Chlorophyll d |
697, 456, 400 |
63.7 |
6.2 |
|
| Chlorophyll f |
707, 440, 398 |
71.1 |
|
|
| Bacteriochlorophyll a |
773, 577, 358 |
90.0 |
2.9 |
0.2 |
| Bacteriochlorophyll b |
791, 592, 372 |
106 |
2.4 |
|
| Bacteriochlorophyll c |
659, 429 |
75 |
6.7 |
0.29 |
| Bacteriochlorophyll d |
651, 423 |
79 |
6.3 |
|
| Bacteriochlorophyll e |
649, 462 |
49 |
2.9 |
|
| Bacteriochlorophyll f |
645, 467 |
|
3.4 |
0.13 |
| Bacteriochlorophyll g |
762, 566, 365 |
76 |
2.7 |
|
aMost data taken from Scheer (1991) or Niedzwiedzki and Blankenship (2010). Solvents are not the same for all quantities.
bValues for ɛ maxare for the longest wavelength absorbing Q yband.
Carotenoids are found in all known native photosynthetic organisms, as well as in many nonphotosynthetic organisms (Britton et al ., 1998; Frank et al ., 2000; Polívka and Frank, 2010). There are many hundreds of chemically distinct carotenoids, so we will not give a comprehensive list. However, there are some consistent structural features that are common to most photosynthetic carotenoids. They are extended molecules with a delocalized π electron system. Carotenoids from oxygenic organisms usually contain ring structures at each end, and most carotenoids contain oxygen atoms, usually as part of hydroxyl or epoxide groups. Structures of several of the carotenoids found in photosynthetic systems are shown in Fig. 4.11.
Carotenoid biosynthesis consists of the building up of large molecules from a basic building block, the five‐carbon branched‐chain species isoprene (Britton et al ., 1998). It is successively condensed into 10‐, 20‐, and 40‐carbon molecules, ending with the compound phytoene. Phytoene is a hydrocarbon consisting of eight isoprene units attached in a linear fashion. It is colorless, because most of the double bonds are isolated. The second stage in the biosynthesis consists of successive desaturation steps, producing a series of intermediates with an increasing number of conjugated double bonds. This has the effect of shifting the absorption into the visible region. The end product of this stage is the compound lycopene, which is responsible for the red color of tomatoes. Some of the intermediates, such as neurosporene, are the end point in carotenoid biosynthesis for some anoxygenic photosynthetic bacteria. In most organisms, there are two additional stages of the biosynthetic pathway: cyclization of the ends of the molecule followed by derivatization by hydroxylation or any of a wide variety of other processes.
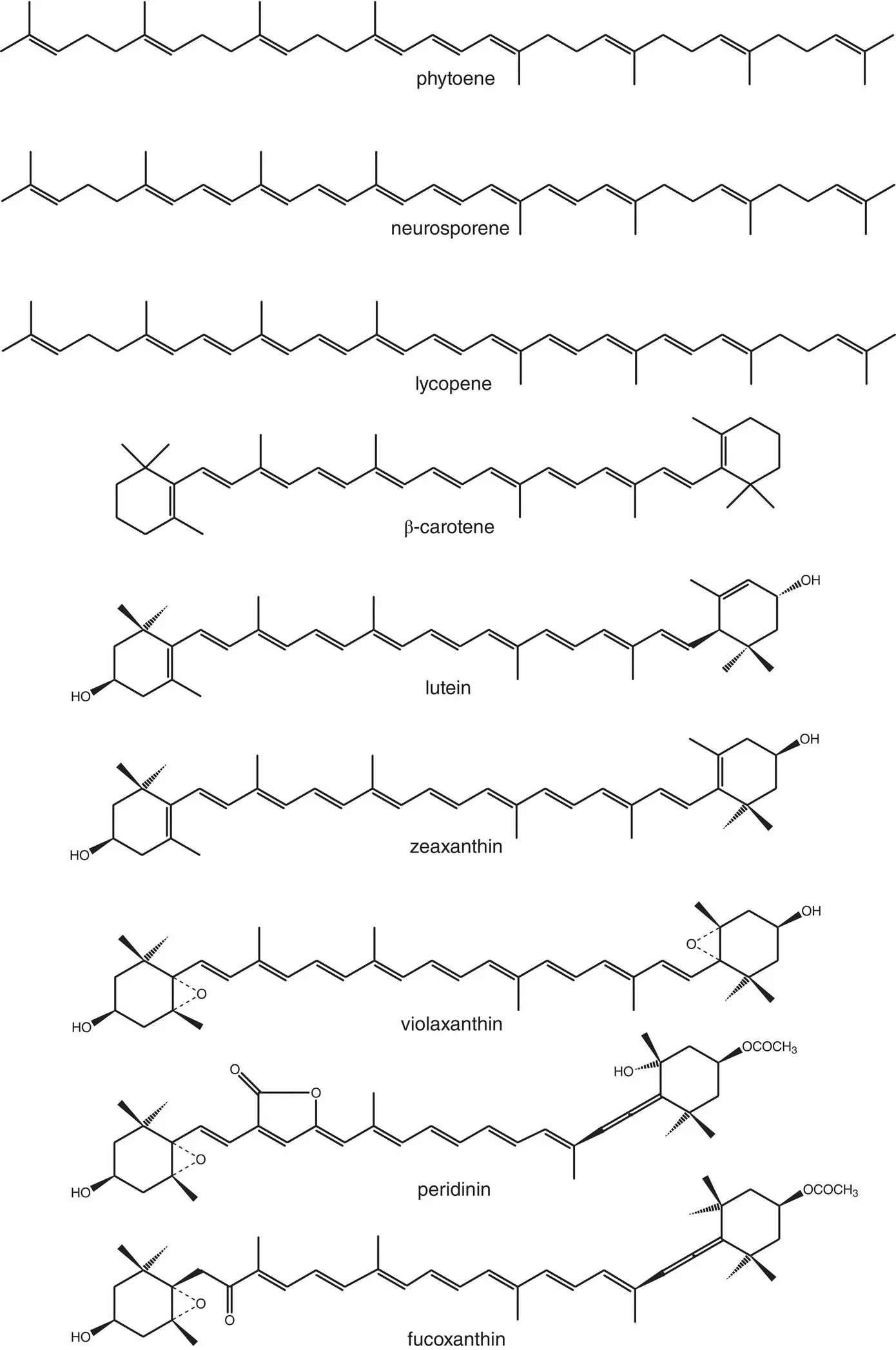
Figure 4.11 Structures of several carotenoids and carotenoid precursors important in photosynthetic systems.
Carotenoids have several well‐documented essential functions in photosynthetic systems. First, they are accessory pigments in the collection of light, absorbing light and transferring energy to a chlorophyll‐type pigment. Most antenna complexes contain carotenoids. Second, carotenoids function in a process called photoprotection. Carotenoids rapidly quench triplet excited states of chlorophylls before they can react with oxygen to form the highly reactive and damaging excited singlet state of oxygen. They also quench the singlet oxygen if it is somehow formed. Finally, carotenoids have recently been shown to be involved in the regulation of energy transfer in antennas. These processes, which avoid overexcitation of the photosynthetic system by safely dissipating excess energy, have different mechanisms in different organisms. We will discuss them in more detail in Chapter 5.
Carotenoids have very unusual energetic and spectroscopic properties. They usually exhibit an intense absorption band, typically in the 400–500 nm range, giving them their characteristic orange color. However, this transition is from the ground state (S 0) to the second excited singlet state (S 2), instead of to the first excited singlet state (S 1). The transition from the ground state to the first excited singlet state is forbidden because of the symmetry of the carotenoid molecule. An energy level diagram typical of many carotenoids is shown in Fig. 4.12. The lifetime of S 2is very short, usually relaxing to S 1by internal conversion on a subpicosecond time scale. This short lifetime of S 2means that fluorescence of the S 2state is highly quenched and is not observed in most situations. From the S 1state, the excited carotenoid can relax to the ground state. However, this relaxation is also almost always nonradiative. The fluorescence decay rate constant of an excited state is related to the strength of the absorption that forms the excited state (Eq. A.68). If an absorption transition is extremely weak, such as the S 0to S 1carotenoid absorption, then the intrinsic fluorescence decay rate constant between these two states will be very small, and fluorescence will make a negligible contribution to the excited state decay (Eq. A.69). Internal conversion from S 1to S 0is typically very efficient, so the S 1state has a picosecond lifetime. Carotenoids with nine or more double bonds have additional dark states in addition to the three energy levels discussed here (Ostroumov et al ., 2013).
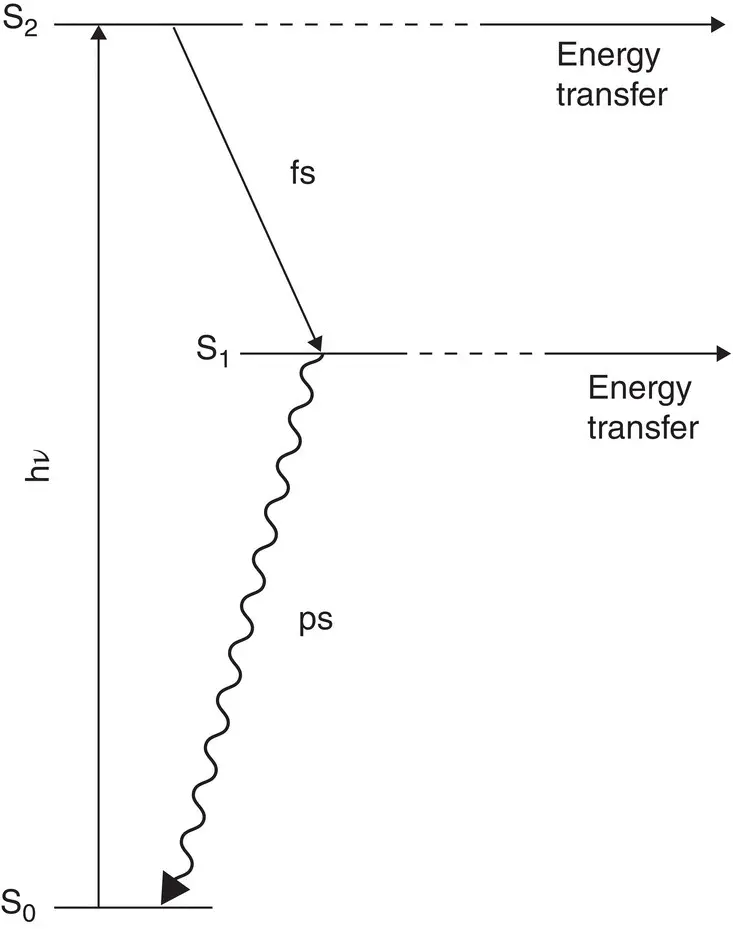
Figure 4.12 Energy Level diagram typical of carotenoids.
The energy of the S 1state of the carotenoid is very difficult to measure directly, because of the forbidden nature of the S 0to S 1transition. One method that can be used to determine this energy is two‐photon spectroscopy, in which two photons are absorbed simultaneously, with the sum of their energies equal to the transition energy. The S 0to S 1transition is allowed under these conditions (Krueger et al ., 1999).
Читать дальше














