By convention, the y molecular axis of all chlorophylls is defined as passing through the N atoms of rings A and C, with the x axis passing through the N atoms in rings B and D. The z axis is perpendicular to the plane of the macrocycle. An extensive delocalized π electron system extends over most of the molecule, with the exception of ring D, in which the C‐17–C‐18 double bond is reduced to a single bond. The tail is formed by condensation of four five‐carbon isoprene units and is then esterified to ring D. It is often called the phytyl tail, after the polyisoprenoid alcohol precursor phytol that is attached during biosynthesis. It is also sometimes called the isoprenoid tail.
Most of the chlorophylls are classified chemically as chlorins rather than porphyrins, by virtue of the reduced ring D. Most of the bacteriochlorophylls are similarly called bacteriochlorins, because of the reduction of both rings B and D. All chlorophylls and bacteriochlorophylls contain the extra ring E, which is called the isocyclic ring.
Most chlorophyll‐type pigments contain three chiral carbon atoms, C‐13 2, C‐17, and C‐18. Bacteriochlorophyll a contains two additional chiral centers, C‐7 and C‐8. In all cases, the stereochemical fidelity of the biosynthetic enzymes is extremely high, so the compounds found in cells are a single species and not mixtures of diastereomers (except as noted below).
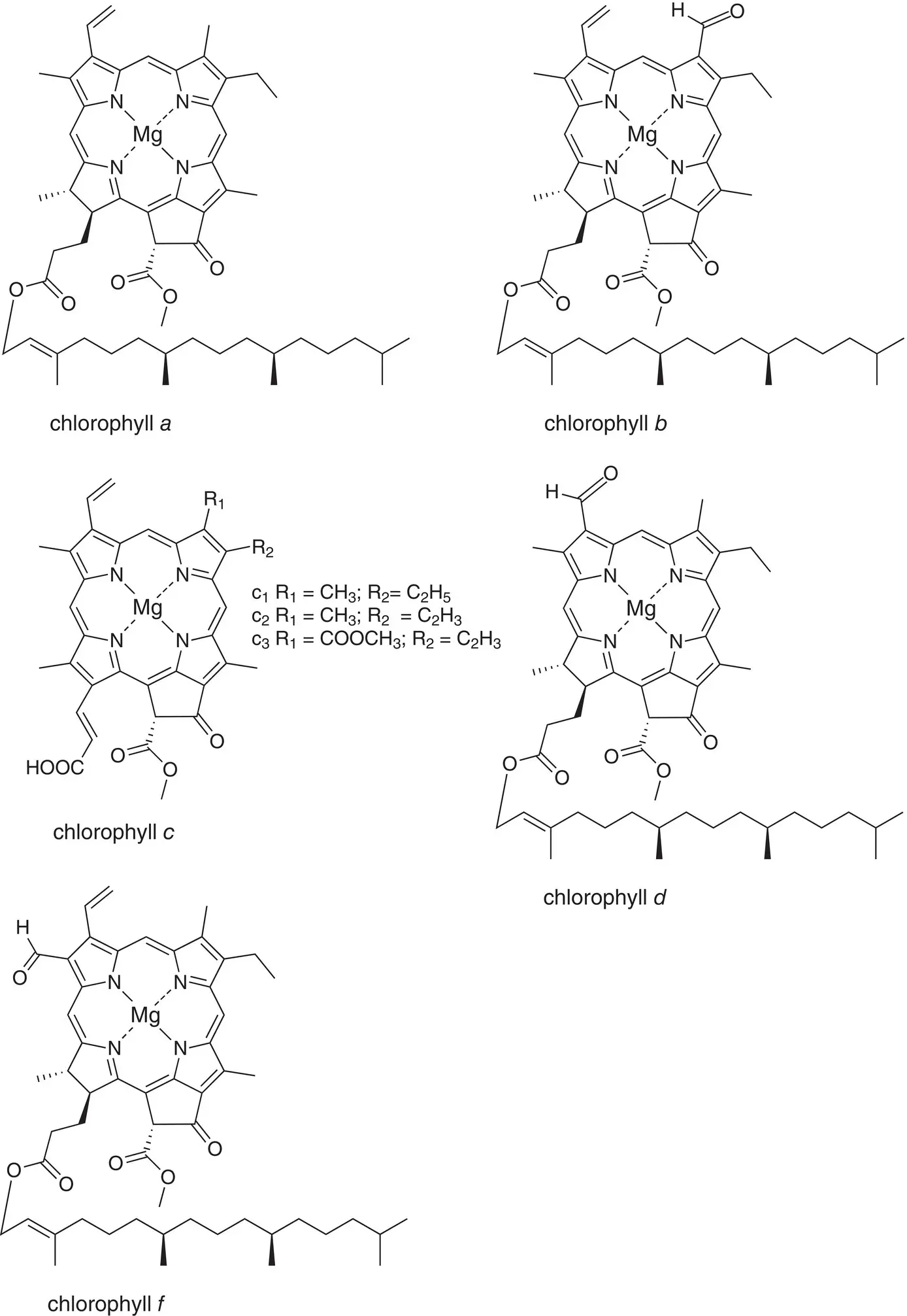
Figure 4.3 Chemical structures of chlorophylls a, b, c, d, and f . R 1, R 2, etc. refer to ring substituents. In some cases, more than one possible group can be found at some positions.
The structures of all major chlorophylls and bacteriochlorophylls are shown in Figs. 4.3and 4.4. The distribution of photosynthetic pigments in different classes of photosynthetic organisms is given in Table 4.1.
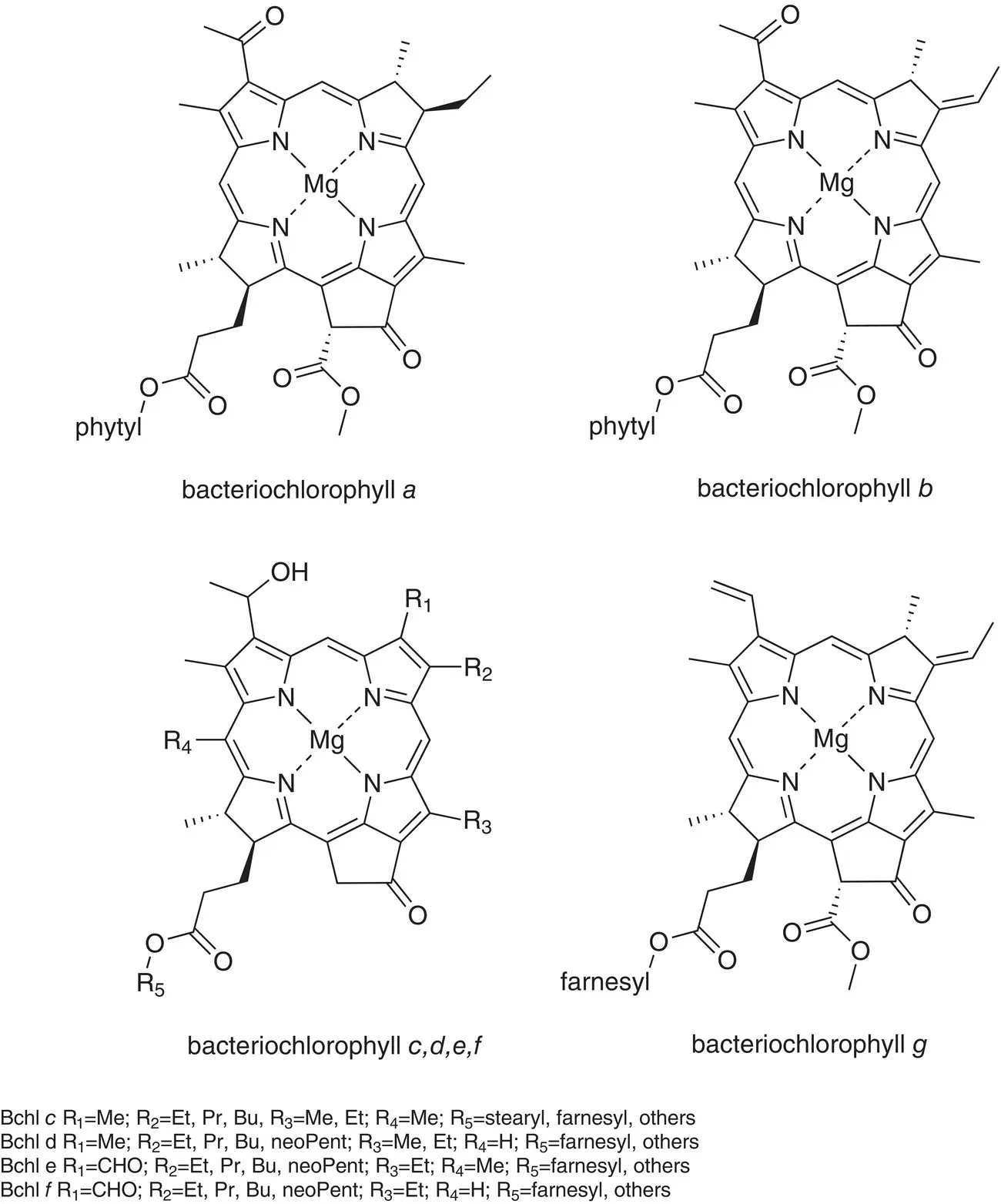
Figure 4.4 Chemical structures of bacteriochlorophylls a, b, c, d, e, f, and g .
Chlorophyll a is found in all known eukaryotic photosynthetic organisms. Among prokaryotes, it is found in large quantities only in the cyanobacteria (including the prochlorophytes), although traces of chlorophyll a or minor variants are found in some anoxygenic bacteria, where it is thought to have an important function as an intermediate in the electron transport chain. Some prochlorophytes contain divinyl chlorophyll a , in which the substituent at the C‐8 position on ring B is vinyl instead of ethyl.
Table 4.1 Distribution of chlorophylls and bacteriochlorophylls
| Type of organism |
Chl a |
Chl b |
Chl c |
Chl d,f |
BChl a |
BChl b |
BChl c,d,e |
BChl g |
Carotenoids |
Bilins |
| Purple bacteria |
|
|
|
|
+ a |
+ a |
|
|
+ |
|
| Green sulfur bacteria |
|
|
|
|
+ |
|
+ |
|
+ |
|
| Filamentous anoxygenic phototrophs |
|
|
|
|
+ |
|
+ |
|
+ |
|
| Heliobacteria |
|
|
|
|
|
|
|
+ |
+ |
|
| Cyanobacteria |
+ |
+ b |
+ b |
+ b |
|
|
|
|
+ |
+ |
| Green algae |
+ |
+ |
|
|
|
|
|
|
+ |
|
| Diatoms |
+ |
|
+ |
|
|
|
|
|
+ |
|
| Brown algae |
+ |
|
+ |
|
|
|
|
|
+ |
|
| Dinoflagellates |
+ |
|
+ |
|
|
|
|
|
+ |
|
| Cryptomonads |
+ |
|
+ |
|
|
|
|
|
+ |
+ |
| Red algae |
+ |
|
|
|
|
|
|
|
+ |
+ |
| Plants |
+ |
+ |
|
|
|
|
|
|
+ |
|
aPurple a or b , but not both in the same species.
bMost cyanobacteria contain Chl a as their only chlorophyll‐type pigment. Prochlorophytes contain in addition Chl b . Some types also contain Chl c , d , or f .
An important variant of chlorophyll a is chlorophyll a ′. This pigment differs from chlorophyll a only in the stereochemistry at the C‐13 2position. It is found in small but reproducible amounts in photosystem I complexes, where one molecule forms half of P700, the special pair of pigments that is the primary electron donor (see Chapter 7). The spectral and redox properties of chlorophyll a ′ are very similar to those of chlorophyll a . Current evidence suggests that chlorophyll a ′ is made from pre‐existing chlorophyll a , although the putative C‐13 2invertase enzyme has not been identified.
Chlorophyll b is identical to chlorophyll a except at the C‐7 position, where a formyl group replaces the methyl group. This change shifts the maximum absorption to shorter wavelengths. Chlorophyll b is the major accessory light‐absorbing pigment in light‐harvesting complexes in the majority of eukaryotic photosynthetic organisms, including plants and green algae, and is not found in reaction center complexes. In photosynthetic prokaryotes, it is found only in the prochlorophytes, in some cases as divinyl chlorophyll b .
Chlorophyll c is perhaps the most unusual of all the chlorophylls, in that it does not have an isoprenoid tail and also does not have ring D reduced. It is therefore chemically classified as a porphyrin, and not as a chlorin. Chlorophyll c is found exclusively in various groups of marine algae, such as diatoms and dinoflagellates. It functions as an accessory light‐harvesting pigment in pigment–protein complexes similar to those involving chlorophyll b in plants and green algae. There are several structural variants of chlorophyll c , which vary in some of the peripheral ring substituents, as shown in Fig. 4.3.
Chlorophyll d is different from chlorophyll a in only one respect: the substituent at the C‐3 position is a formyl group in chlorophyll d , instead of the vinyl group found in chlorophyll a . For many years, chlorophyll d was known only as a trace constituent of certain red algae and was suspected to be an experimental artifact. However, in 1996, a cyanobacterium, Acaryochloris marina, was discovered as a symbiont in a marine animal called an ascidian. This organism contains chlorophyll d as the major pigment, although it also contains small amounts of chlorophyll a . It now appears that chlorophyll d is only found in cyanobacteria, and that earlier reports of chlorophyll d in red algae result from contamination of the algal surface by epiphytic cyanobacteria that contain chlorophyll d (Larkum and Kuhl, 2005). The electron withdrawing formyl group at the C‐3 position adjacent to the y molecular axis of the pigment has the effect of shifting the absorption maximum of chlorophyll d to longer wavelengths compared to chlorophyll a . It has therefore received attention as a possible pigment to expand the solar spectrum in bioenergy applications (Chen and Blankenship, 2011).
Читать дальше














