Source: Beale (1999) (p. 45)/Springer Nature.
Chlorophyll b is made from chlorophyll a by oxidation of the methyl group at C‐7 to give a formyl group, using a mixed‐function oxidase enzyme that depends on O 2(Tanaka and Tanaka, 2007). Chlorophyll b can also be reduced back to chlorophyll a . The ratio of the two pigments is tightly regulated and can be adjusted as needed.
4.4 Spectroscopic properties of chlorophylls
The chlorophylls all contain two major absorption bands, one in the blue or near UV region and one in the red or near IR region ( Fig. 4.7). The blue absorption band produces a second excited state that very rapidly (picoseconds) loses energy as heat to produce the lowest excited state. The lowest excited state is relatively long‐lived (nanoseconds) and is the state that is used for electron transfer and energy storage in photosynthesis.
The lack of a significant absorption in the green region gives the chlorophylls their characteristic green or blue–green color. These absorption bands are π → π * transitions, involving the electrons in the conjugated π system of the chlorin macrocycle. The absorption and fluorescence spectra of chlorophyll a and bacteriochlorophyll a are shown in Fig. 4.8. The two lowest‐energy transitions are called the Q bands, and the two higher‐energy ones are known as the B bands. They are also commonly called Soret bands.
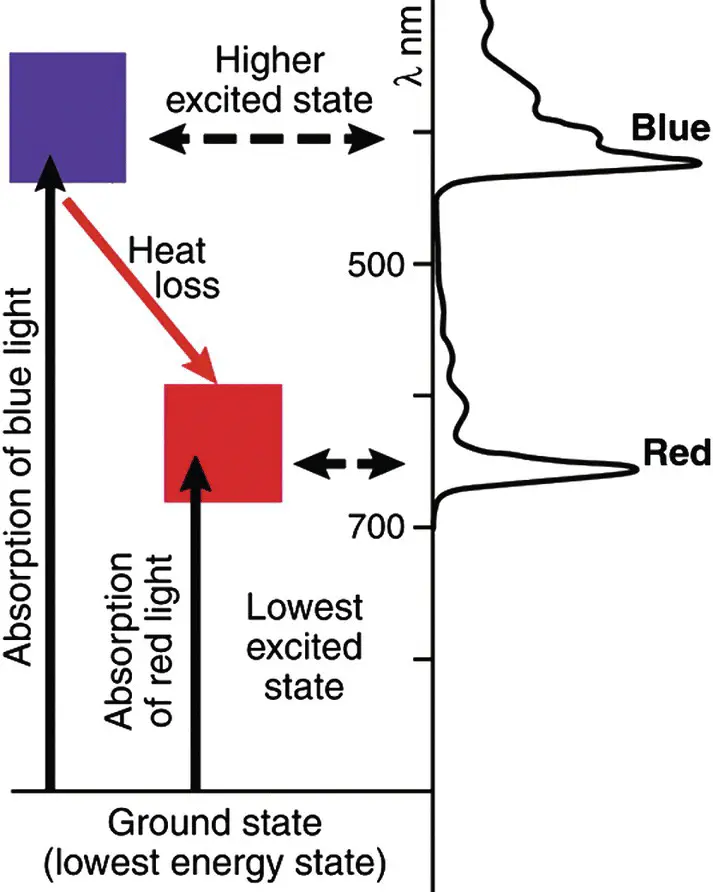
Figure 4.7 Absorption spectrum and simplified energy level diagram for chlorophyll a . The blue absorption band populates a second excited state that rapidly converts to the lowest excited state that can also be populated by absorption of a red photon. Energy storage in photosynthesis always occurs from the lowest excited state and the energy difference between the higher excited states and the lowest excited state is lost as heat.
Source: Blankenship et al . (2011) (p. 807)/American Association for the Advancement of Science.
The spectra can be described theoretically by using a “four orbital” model, originally proposed by Martin Gouterman (1961). The four π molecular orbitals that are principally involved in these transitions are the two highest occupied molecular orbitals (HOMOs) and the two Lowest Unoccupied Molecular Orbitals (LUMOs). The molecular orbital picture and the one‐electron transitions predicted for a symmetric porphyrin, chlorin, and bacteriochlorin are shown in Fig. 4.9.
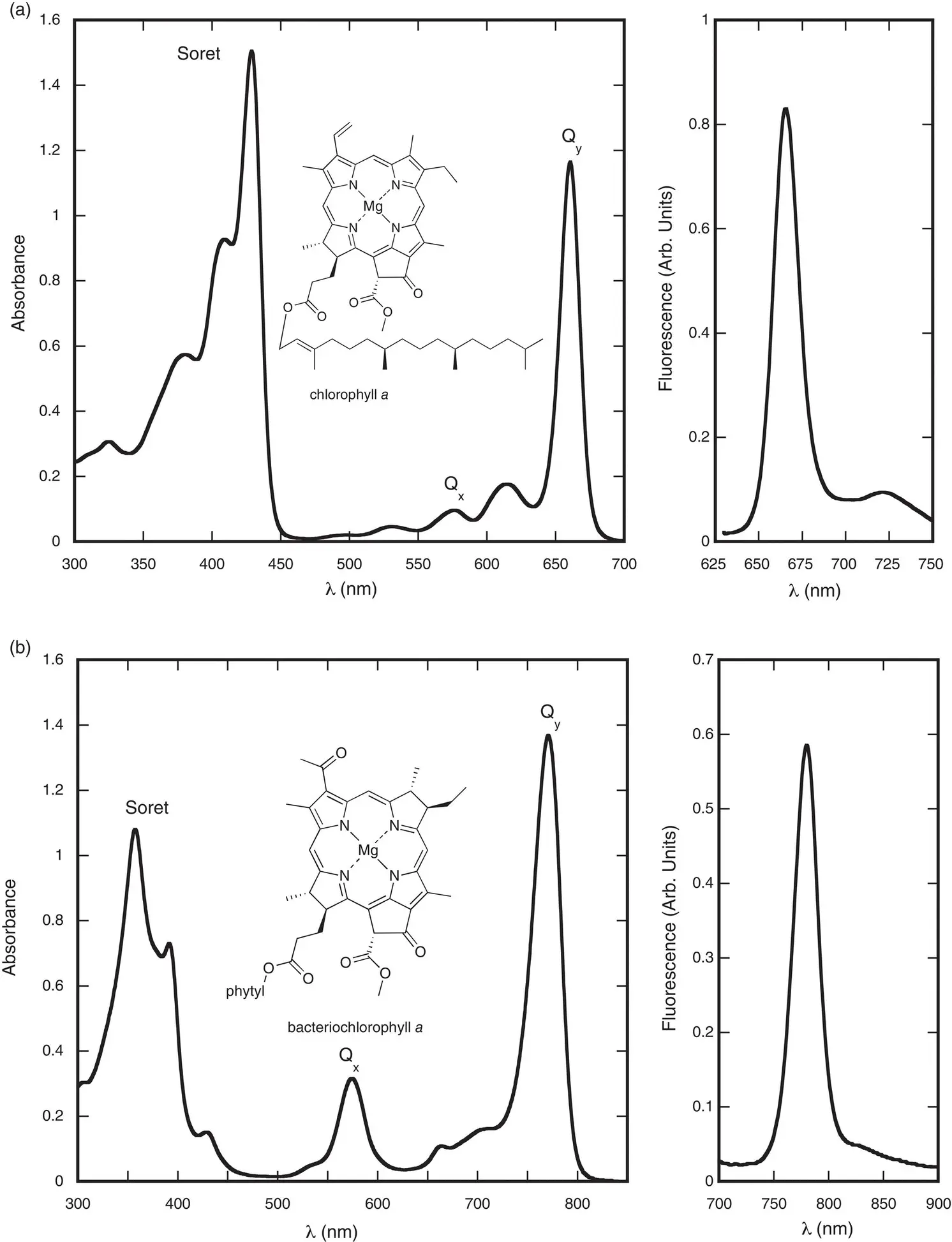
Figure 4.8 Absorption (left) and fluorescence (right) spectra of (a) chlorophyll a and (b) bacteriochlorophyll a in diethyl ether.
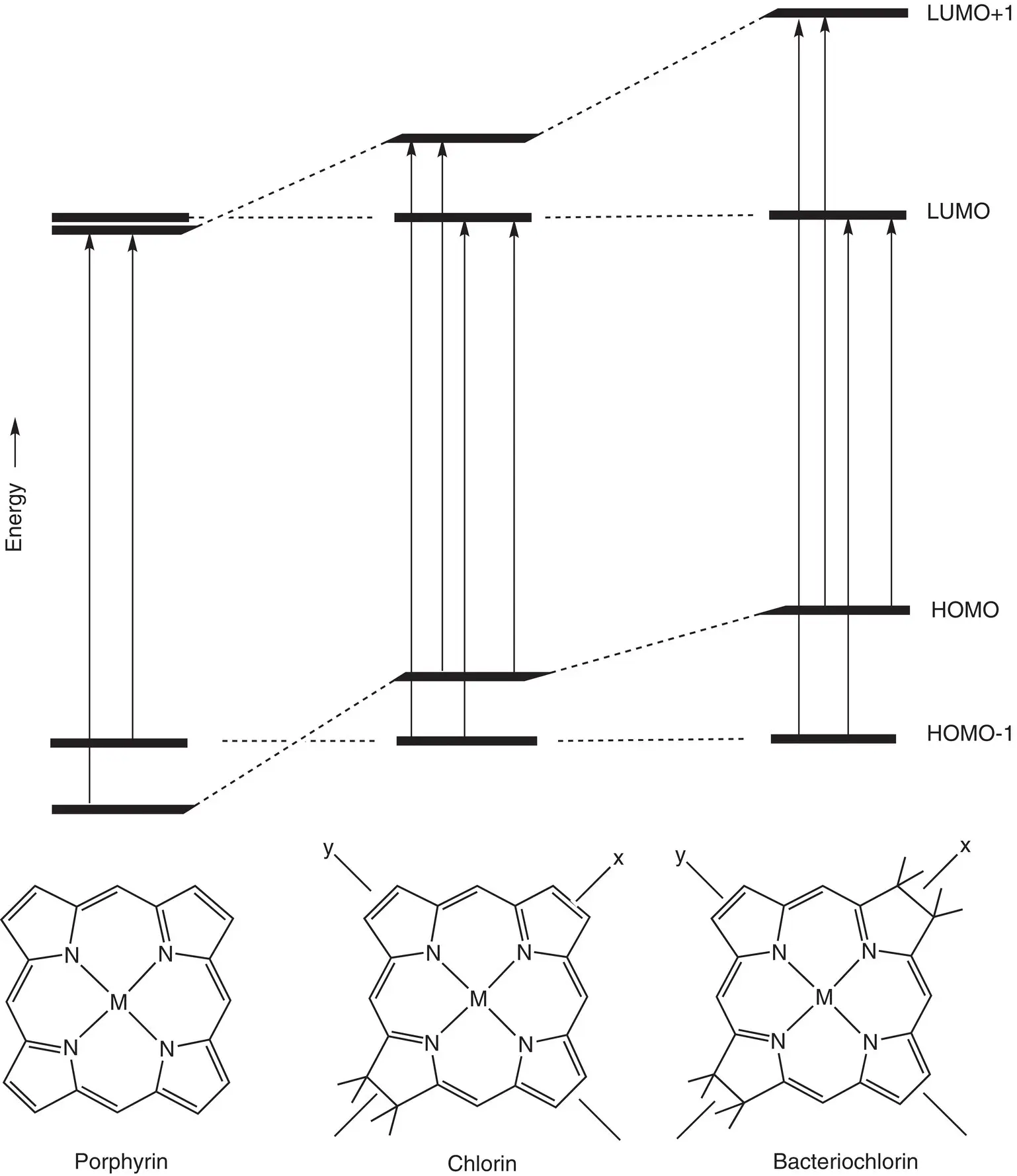
Figure 4.9 Molecular orbital energy level diagram of porphyrin, chlorin, and bacteriochlorin with one‐electron transitions that combine via configuration interaction to give the different electronic transitions indicated by arrows.
The diagram shown in Fig. 4.9is an oversimplification of what is a complex relationship between electronic states and orbital energies. It is not correct to conclude that electronic transitions reflect a simple promotion of an electron from a HOMO to a LUMO. In reality, several different electronic configurations, including contributions from much higher‐energy molecular orbitals can contribute to the electronic transition. This phenomenon is known as configuration interaction. The result is that there is not a simple one‐to‐one correspondence between orbital occupations and electronic transitions. However, it is also the case that the majority contribution to the transition can come from a single configuration. This becomes more correct as one goes from the more symmetric porphyrin to the very asymmetric bacteriochlorin, so that the Q yabsorption band in bacteriochlorophyll is described reasonably well by the simple HOMO to LUMO transition, while this is not valid for the more symmetrical porphyrin. The energies and electron densities associated with the four frontier molecular orbitals for a Zn‐chlorin are shown in Fig. 4.10. The one‐electron transitions that combine via configuration interaction to produce the excited electronic state are shown as arrows, with the major transitions that combine to produce the Q ytransition labeled Y and those that combine to produce the Q xtransition labeled X.
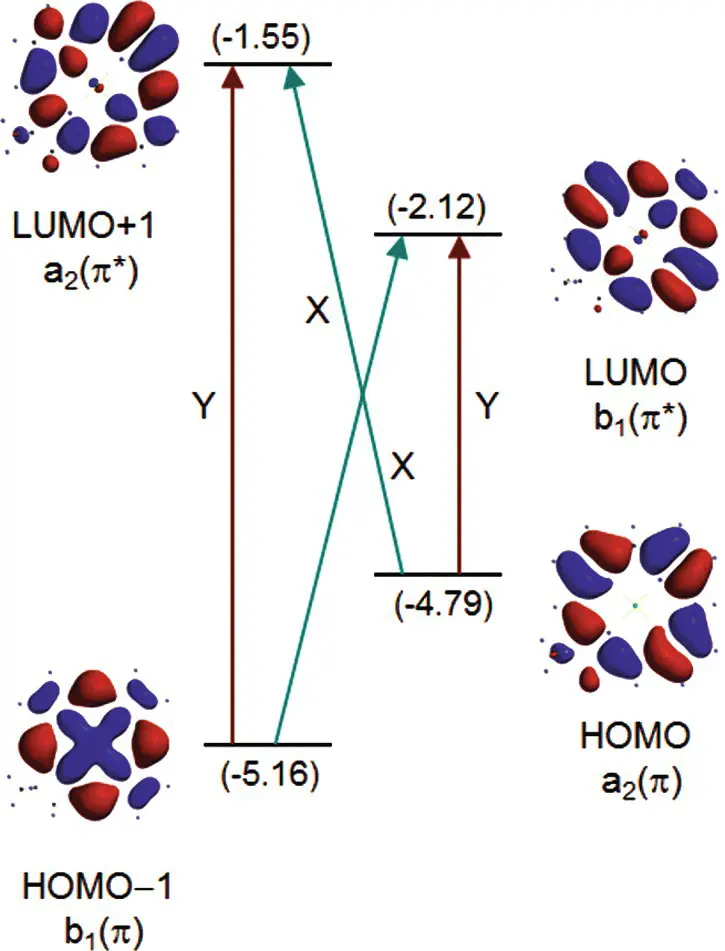
Figure 4.10 Schematic orbital energy diagram for a Znchlorin, illustrated by the calculated orbital energies (in eV) and electron density distributions. The diagram also shows the one‐electron promotions that give rise to the electronic states via configuration interaction according to the four‐orbital model.
Source: Kee et al . (2007) (p. 1137)/John Wiley and Sons.
The electronic transitions have transition dipole moments with different strengths and orientations. The longest‐wavelength transition is invariably polarized along the y ‐axis of the molecule and is therefore known as the Q ytransition. The y ‐axis extends from the N atom in ring A to the N atom in ring C ( Fig. 4.2). This means that the absorption will be strongest if the electric vector of plane‐polarized exciting light is parallel to the molecular axis of the pigment. The exciting light couples to the π electrons of the molecule and rearranges them somewhat during the transition. The Q ytransition causes a shift in electron density that is directed along the y molecular axis of the molecule. In a similar fashion, the x ‐axis extends from the N atom in ring B to the N atom in ring D.
For both chlorophyll a and bacteriochlorophyll a , the Q ytransition is strongly polarized along the y molecular axis (see Appendix). The weaker Q xtransition in bacteriochlorophyll is also strongly polarized along the x molecular axis. The Q xtransition in chlorophyll, however, is not as well resolved as in bacteriochlorophyll, and theoretical calculations suggest that it is not polarized directly along the x molecular axis. The Soret bands have a mixed polarization.
In addition to the fundamental Q and B electronic transitions, vibrational overtone transitions can also be observed, especially on the Q yband. These represent a simultaneous vibrational and electronic transition, with the final state being an excited vibrational state of the excited electronic state. A progression of vibrational states can be observed, with the most intense transition the 0,0 band, with the higher energy satellites termed 0,1; 0,2; and so on. The first number is the vibrational quantum number of the ground electronic state before light absorption, and the second number the vibrational state of the excited electronic state after the transition. Of course, there are many vibrations in a chlorophyll molecule, and only one of these is the one responsible for the vibrational structure in the absorption spectrum.
The fluorescence spectrum of all chlorophylls peaks at slightly longer wavelengths than the absorption maximum. The fluorescence emission is polarized along the y molecular axis, as it is emitted from the Q ytransition. The fluorescence spectrum usually has a characteristic “mirror image” relationship to the absorption. This is because the ground and excited states have similar shapes, so those molecular vibrations that are activated during electronic absorption are also likely to be activated upon fluorescence emission. However, in this case, the initial state is the ground vibrational state of the excited electronic state, and the final state is the excited vibrational state of the ground electronic state. This causes a shift of the emission to the longer‐wavelength side of the main transition, in what is known as the Stokes shift(see Appendix).
Читать дальше
















