Commercially available gentamicin‐impregnated collagen sponges have been placed intraarticularly in a study population of dogs with sterilely induced synovitis. A rapid elution rate was identified, resulting in a rapid rise of intraarticular gentamicin concentrations, but a rapid decline was also observed. This rapid decline may not be clinically relevant, as gentamicin is a concentration‐dependent antimicrobial and thus the high concentrations achieved may be sufficient to result in bactericidal activity.
The gentamicin‐impregnated sponge was found to incite joint inflammation, lameness, and renal impairment and was thus not recommended for clinical use [30]. However, one case report exists of its use in a clinical incidence of septic arthritis following an extracapsular stifle stabilization procedure. This case reports clinical resolution of the septic arthritis but systemic gentamicin was administered simultaneously, making it hard to determine the role of the intraarticular gentamicin [31]. A separate case series combined implant removal with an amikacin‐impregnated collagen sponge being placed at the site of implant removal for treatment of TPLO SSI, which resulted in a 96.8% long‐term resolution rate [32]. The benefit of an antimicrobial collagen sponge is its absorbable nature, thus precluding the requirement for retrieval following SSI resolution.
While all of these local antimicrobial‐eluting options provide increased levels of local therapy, differences in biodegradability may play a role in clinical decision making. If these local therapies are being employed in the face of an incompletely healed osteotomy or incomplete stabilization of an extracapsular repair, implant removal cannot be performed concurrently. Two sides of the coin can therefore be considered following complete healing of the osteotomy or adequate stifle stabilization. On the one hand, a biodegradable option does not require further surgical extraction and if the SSI is clinically resolved, the implant may not require removal. However, on the other hand, a nonbiodegradable option must be surgically removed and allows the opportunity for concurrent implant removal. With the major concern of biofilm formation on implanted materials, locally applied antimicrobial therapy may not be sufficient to eradicate the SSI and recurrence may result in further treatment and possible implant removal at a later date.
In the case of implant removal, the major microorganism burden is removed with the implant, assuming that most of these SSIs are biofilm related and as such systemic or local therapies may not be required ( Figure 3.6). Of the various studies reporting treatment of SSIs with implant removal, few indicate whether postoperative systemic antimicrobial therapy was continued empirically and/or altered pending culture and susceptibility testing results. While all these studies encourage culture and susceptibility testing of implants that are removed, how this information is applied clinically is unknown. As such, evidence‐based recommendations for postoperative systemic antimicrobial use following implant removal for management of an SSI remain unclear. Logically, if the major burden of disease has been removed, monitoring for clinical recurrence of SSI signs is recommended. Thus, patients without active evidence of an SSI at the time of implant removal do not require systemic antimicrobial therapy and results of culture and susceptibility testing should be employed only in the face of recurrent signs of SSI.
Though few studies exist, when local antimicrobial therapy is combined with implant removal, such as implantation of an amikacin‐impregnated collagen sponge or amikacin and clindamycin polymer gel, SSI resolution rates ranged from 95% to 96.8%, without the concurrent use of systemic antimicrobials [29, 32].
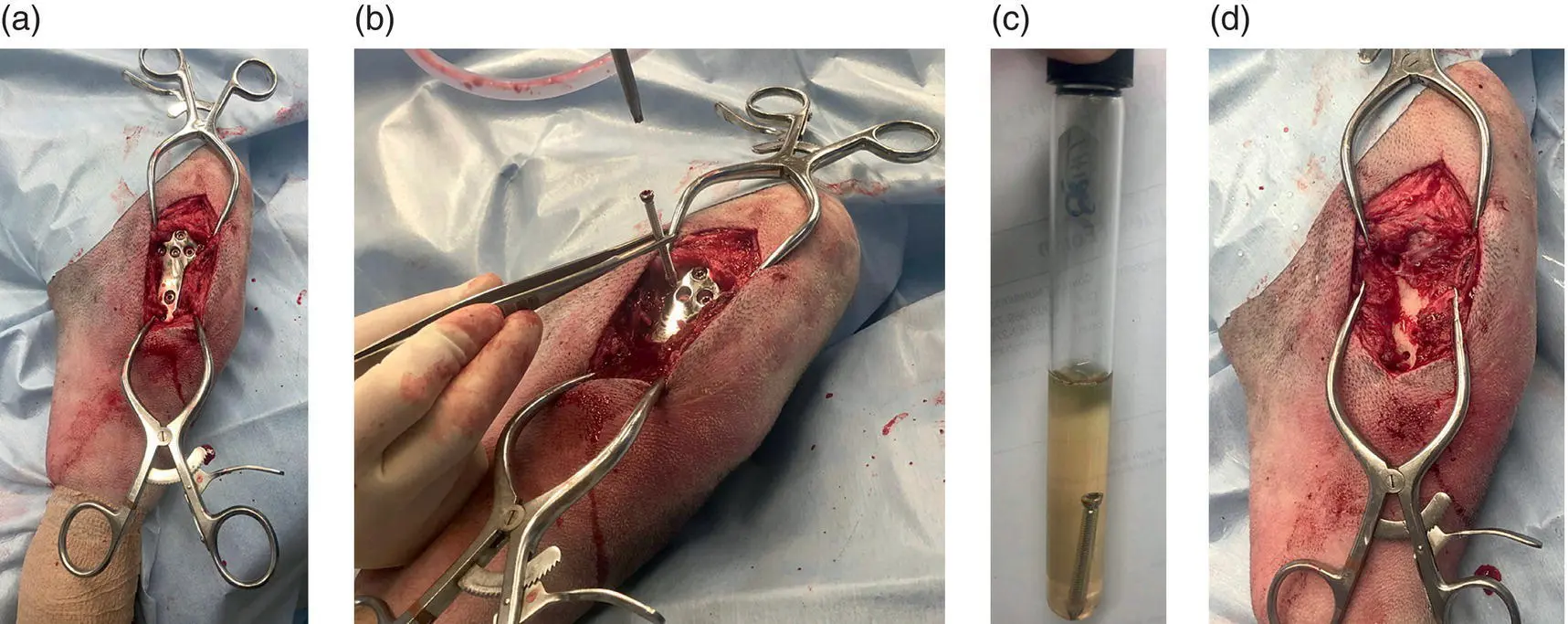
Figure 3.6 (a)Exposed TPLO plate. (b)Screw removed. When removing screws to submit for culture, use a pair of forceps that have not touched the skin to stabilize the screw while it is being removed. Do not allow the screw to contact the skin to avoid growth of skin contaminants on your bacterial culture. (c)Screw placed in broth medium for bacterial culture submission. (d)Surgical site following implant removal.
Regardless of local or systemic therapies used in conjunction with implant removal, bacterial culture and susceptibility testing is recommended following implant removal. While a positive culture may not indicate the need for antimicrobial treatment, it allows for proper selection of the antimicrobial if treatment is determined to be clinically indicated. A positive culture result has been reported following implant removal in 72–80% of cases [18, 23]. However, sonication of implants may aid in improved bacterial recovery when considering culture and susceptibility testing [33]. As sonication is not likely to be performed routinely for culture and susceptibility testing, this may play a role in identification of negative culture results following implant removal for SSI. Alternatively, preoperative treatment with antimicrobial therapy targeted at the known microorganisms may be sufficient to eradicate or significantly reduce the microbial burden, such that a negative culture result is achieved post implant removal [13, 18, 23].
Following diagnosis of an SSI, continued frequent communication is necessary to ensure response to therapy. If empirical therapy is instituted without improvement of clinical signs, bacterial culture and susceptibility testing is recommended to guide therapy, if this is not already under way. Duration of antimicrobial therapy is controversial but should reflect the level of the infection, such that a superficial SSI receives a shorter course of antimicrobial therapy than a deep or organ/space SSI. At any time that antimicrobial therapy has been discontinued and clinical signs of SSI recur, implant removal should be considered to remove the largest burden of disease associated with a likely biofilm‐associated implant infection. Following discontinuation of antimicrobial therapy or implant removal, active surveillance protocols to acquire follow‐up information regarding resolution or recurrence of clinical signs should be implemented. Setting in place an infection control plan in your hospital to determine annual SSI rates in general, but specifically associated with surgically implanted devices, will be beneficial to document and monitor trends, thus allowing earlier interventions when SSI rates are increasing.
1 1. Centers for Disease Control and Prevention (2020). Centers for disease control and prevention: surgical site infection (SSI) event.
2 2. Wolf, R.E., Scavelli, T.D., Hoelzler, M.G. et al. (2012). Surgical and postoperative complications associated with tibial tuberosity advancement for cranial cruciate ligament rupture in dogs: 458 cases (2007–2009). J. Am. Vet. Med. Assoc. 240 (12): 1481–1487.
3 3. Frey, T.N., Hoelzler, M.G., Scavelli, T.D. et al. (2010). Risk factors for surgical site infection‐inflammation in dogs undergoing surgery for rupture of the cranial cruciate ligament: 902 cases (2005–2006). J. Am. Vet. Med. Assoc. 236 (1): 88–94.
4 4. Turk, R., Singh, A., and Weese, J.S. (2015). Prospective surgical site infection surveillance in dogs. Vet. Surg. 44: 2–8.
5 5. Löfqvist, K., Kjelgaard‐Hansen, M., and Nielsen, M.B.M. (2018). Usefulness of C‐reactive protein and serum amyloid a in early detection of postoperative infectious complications to tibial plateau leveling osteotomy in dogs. Acta Vet. Scand. 60 (30): 1–8.
Читать дальше