Regardless of the potential for resistance, response to topical therapy should be monitored and treatments altered if there is a lack of response. Ideally, a bacterial culture should be submitted from the onset of clinical signs, so that an appropriate systemic antimicrobial can be added to the treatment regime, should topical therapy fail to resolve the SSI.
Systemic antimicrobial therapies should be employed in the face of an intact incision and direct identification of microorganisms. Initially, systemic antimicrobials will be chosen empirically until the results of culture and susceptibility testing are available to guide specific therapy. It is important to collect and submit a bacterial culture when an SSI is suspected, to ensure appropriate antimicrobial stewardship is followed and reduce the risk of potentiating antimicrobial resistance.
Initial empirical recommendations will vary based on hospital known infectious agents and their resistance patterns. Appropriate duration of therapy is a topic of debate and varies between prescribers, with reported treatment lengths ranging from 1 to 6 weeks of antimicrobial therapy [12, 13]. While extended durations of antimicrobial therapy are reported, these prolonged courses of antimicrobials are not likely required unless implanted materials are involved. Most uncomplicated, superficial SSIs will resolve with a systemic course of antimicrobials for 3–5 days. Ultimately, when antimicrobial therapy is discontinued and there is recurrence of clinical signs, involvement of implanted materials should be considered and prolonged antimicrobial therapy based on culture and susceptibility results will be required until implanted materials can be removed or local therapies can be employed [12].
Deep SSIs involving implanted materials can be more challenging to manage, due to the risk for biofilm formation. A biofilm is a community of sessile bacteria embedded in a self‐produced matrix that has adhered to a surface ( Figure 3.4) [14]. The most commonly implicated bacterium in veterinary SSIs is S. pseudintermedius which has a propensity for biofilm formation and multidrug resistance [9, 15, 16]. The self‐produced matrix protects the bacteria from antimicrobial penetration and makes treatment more complex as minimum inhibitory concentrations are much higher for bacteria in a biofilm than planktonic bacteria [17].
Because of this increased challenge of eradicating a biofilm‐associated infection, implant removal is often recommended. However, if the SSI is identified early postoperatively and insufficient healing has occurred, methods other than implant removal must be considered. Antimicrobials are often used in situations like this to try to control the SSI, with a realization that the goal is most likely to allow for bone healing, at which point implant removal would be indicated. In the face of a completely healed osteotomy or adequately stabilized extracapsular repair, implant removal, and thus biofilm eradication, are often recommended. Several studies have reported implant removal rates for TPLO, tibial tuberosity advancement (TTA), cranial closing wedge osteotomy, triple tibial osteotomy, and lateral fabellar suture extracapsular repairs [12, 13,18–23]. Explant rates for management of an SSI ranged from 1.3% to 71%, with TPLO and lateral fabellar sutures being the highest rate of explanted materials [12, 13,18–20, 22, 23].

Figure 3.4 Scanning electron microscopy image of a biofilm.
Source: Adapted from Singh et al. [9].
Additional approaches to biofilm infections are currently limited. Two enzymes have been identified as being able to prevent biofilm formation; deoxyribonuclease I and dispersin B. While both are capable of inhibiting biofilm formation, only dispersin B has been proven to be able to disrupt an established biofilm [24]. A recent in vitro study has identified that the combination of dispersin B and amikacin in a biodegradable gel allows for rapid elution of dispersin B with a gradual reduction in concentrations over a period of 10 days [25]. While this is a promising avenue, clinical application has not yet been assessed.
When implant removal cannot yet be performed, local antimicrobial therapies ( Table 3.3) may be employed. The main goal of these therapies is to allow higher antimicrobial concentrations to be achieved locally than could be tolerated systemically, with limited systemic exposure [26]. Alternative local therapies may include antimicrobial‐eluting bone cement, gel polymer, or collagen sponges, with the most common antimicrobials used being gentamicin, amikacin, and clindamycin.
Table 3.3 Pros and cons of implantable antimicrobial elution products.
| Product |
Pros |
Cons |
| PMMA beads |
Readily available (especially if used for other orthopedic procedures in your facility) |
Nonbiodegradable Must be removed Surgical implantation |
| Calcium‐based beads |
Biodegradable – no removal required |
Surgical implantation |
| Polymer gel |
Can combine with dispersin B to break down biofilm Minimally invasive application – injectable Biodegradable – no removal required |
Cannot flush surgical site to dilute microbial burden Location of application less precise |
| Collagen sponge |
Biodegradable – no removal required |
Surgical implantation Incites inflammation Causes lameness with IA application Causes renal impairment |
IA, intraarticular; PMMA, polymethylmethacrylate.
Bone cement consisting of a calcium base or polymethylmethacrylate (PMMA) may be formed into beads containing various antimicrobials and may be placed locally at the surgical site ( Figure 3.5). The antimicrobial powder or liquid solution is added to the PMMA powder before the addition of the methylmethacrylate liquid. It is important that the antimicrobial of choice will remain stable during the exothermic reaction that takes place when the PMMA powder and methylmethacrylate liquid are combined [26]. Once combined, beads can be formed around a small gauge cerclage wire, to create a string of beads. In vitro evaluation identified that gentamicin‐susceptible methicillin‐resistant S. pseudintermedius (MRSP) was effectively treated with gentamicin‐impregnated beads, whereas gentamicin‐resistant MRSP was not effectively treated and silver‐impregnated beads had no effect on MRSP biofilms [11]. PMMA beads, however, are not biodegradable and require removal at a later date, whereas calcium‐based beads can be degraded locally, thus not requiring removal [27]. Therefore, when considering antimicrobial‐impregnated beads, one must determine the susceptibility of the microorganisms to effectively choose an antimicrobial and take into consideration the potential requirement for removal of beads if PMMA is chosen.
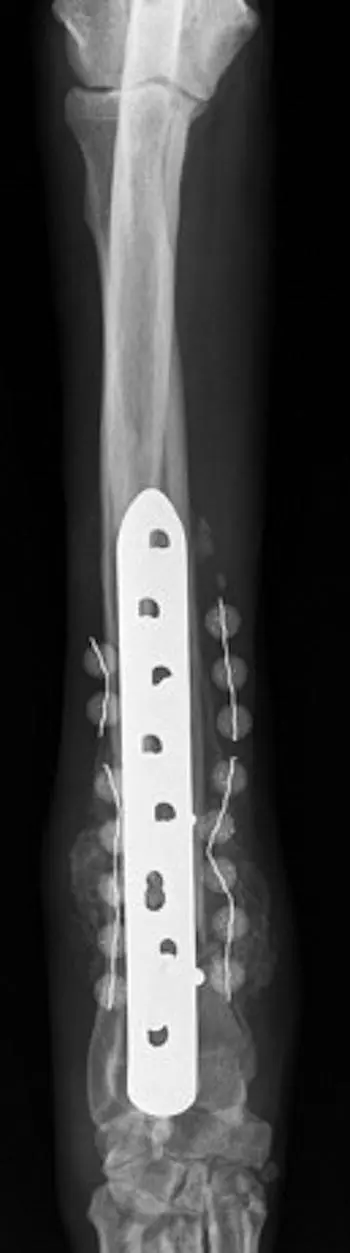
Figure 3.5 In this craniocaudal view of the antebrachium, two discontinuous strands of antimicrobial‐impregnated PMMA beads can be seen on the lateral and medial aspects of the bone.
Antimicrobial‐impregnated dextran polymer gel has been proven in vitro to elute high concentrations of antimicrobials that maintain their bioactivity after elution [28]. Gels can be injected locally, without requiring a surgical approach, and do not require removal as they are naturally resorbed [28]. A single case series of an amikacin and clindamycin‐impregnated dextran polymer gel used in combination with explantation of TPLO plates resulted in excellent clinical outcomes after 12 weeks [29]. Despite limited data existing at this time, this may be a promising option for the future and application without implant removal should be further evaluated.
Читать дальше