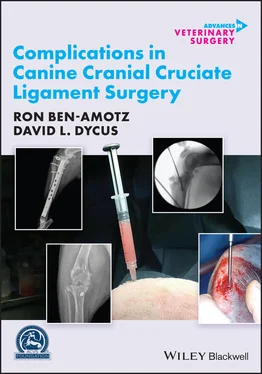
Figure 2.6 Example of a surgical safety checklist, based on the World Health Organization template found at www.who.int/patientsafety/topics/safe‐surgery/checklist/en/.
When considering proximal tibial osteotomy procedures, a variety of plates exist for stabilization of the osteotomy. Several studies have identified increased SSI risk associated with a variety of plates used specifically for TPLOs. Savicky et al. noted an increased SSI rate with Synthes plates whereas Thompson identified an increased SSI risk with Slocum implants [31, 34]. Solano et al. identified an increased risk associated with nonlocking plates compared to locking plates, whereas Giannetto found no significant difference in SSI rates between locking and nonlocking implants [28, 70]. Another study found no significant difference in SSI rates when comparing nonlocking plates, standard locking plates, and double locking plates used in animals >50 kg [19]. As many other factors contribute to the risk of developing an SSI, no one implant associated with TPLO has been shown to be superior at reducing this risk.
The majority of S. pseudintermedius isolates have been identified to be strong to moderate biofilm producers, with no difference between methicillin‐resistant and methicillin‐susceptible forms [71]. A biofilm is a sessile community of bacteria embedded in a self‐produced matrix which is adhered to a surface [72]. With the inherent ability to produce a biofilm, S. pseudintermedius can create an implant‐associated biofilm, making it more difficult for targeted antimicrobial treatment as minimum inhibitory concentrations are much higher for biofilm bacteria than planktonic bacteria [73]. As such, implant coatings such as silver have been assessed for their ability to resist biofilm formation. Silver‐coated implants are excellent at resisting MRSP biofilm formation in vitro , but in vivo evidence is not yet available in veterinary medicine [74]. Other implant coatings for biofilm prevention include heparin as an antiadhesive, chitosan as an antimicrobial, and hydroxyapatite which inhibits biofilm formation while encouraging bone growth [75]. None of these coated implants is used widely in veterinary medicine, nor are there reports of clinical use. Therefore, this may be an avenue for further clinical research as antimicrobial resistance becomes increasingly prevalent.
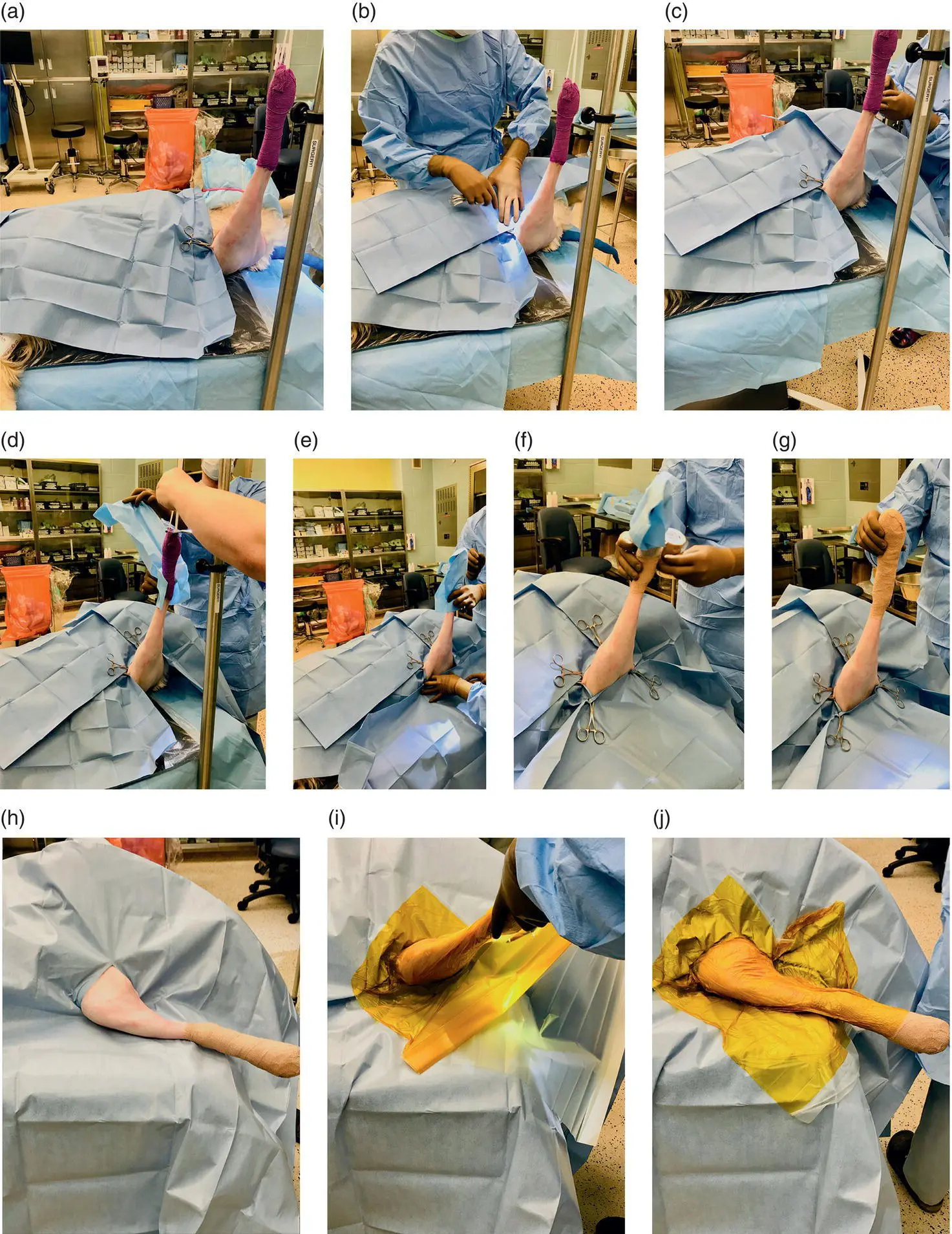
Figure 2.7 Following final skin preparation in the OR with the limb suspended, draping is performed by the sterile surgical team using a four‐quarter technique and top drape, with an optional iodinated incise film. (a)The cranial quarter drape is applied first. (b)A lateral quarter drape applied second. (c)The caudal quarter drape is applied next. (d)A sterile, nonpermeable dressing is used to grasp the nonsterile Vetrapped distal limb, while a nonsterile assistant cuts the tape suspending the limb. (e)While the sterile dressing is applied to the nonsterile distal limb, the final quarter drape is applied. (f)Sterile Vetrap is applied from proximal to distal around the sterile covered distal limb to adhere the sterile dressing in place. (g)Final sterile covering of the distal limb. (h)A top drape is applied over the four‐quarter drapes. (i)An iodinated incise film is applied to the skin. This step is optional. (j)Final appearance of draping.
2.4.5 Wound Closure and Protection
Surgical wound closure and protection play a controversial role in SSI development. Surgical staples in comparison to intradermal skin closures have been reported to increase, decrease or not have any effect on surgical site inflammation and/or SSI development [6, 30, 32]. Skin closure using absorbable skin staples has shown no benefit over stainless steel surgical staples in reducing SSI development [20]. With inconclusive evidence to suggest superiority of one closure method over another, considerations such as time for closure, associated tissue trauma, and potential irritation to the patient must play a role in decision making. While staples may reduce the closure time and thus overall surgery time, this is likely to be negligible for an experienced surgeon. Tissue trauma may also be reduced with skin staples due to minimal manipulation required for placement, but increased inflammation has been noted when surgical staples are used compared to intradermal sutures [6]. Finally, the noncompliant nature of skin staples may lead to increased local irritation and thus potential for self‐trauma from licking or chewing at the surgical site, if not adequately protected from access.
Suture has the proven propensity for biofilm formation and as such may act as a nidus for SSI development [76]. Antimicrobial‐coated suture, specifically triclosan‐coated suture, has been assessed in vitro and in vivo for its ability to resist or reduce SSI development. In vitro , triclosan‐coated monofilament suture had the least bacterial adherence compared to other monofilament and braided suture materials and had greater inhibition for S. pseudintermedius , including MRSP, than Escherichia coli [77, 78]. In vivo , the use of triclosan‐coated monofilament suture was not shown to significantly reduce the risk of SSI in one hospital, while its use contributed to a reduction in SSI rates in another hospital [32, 64]. Further investigation of the use of antimicrobial‐coated suture is warranted. While a true benefit to antimicrobial‐coated suture remains unclear, the only limitation to its use is its increased cost, and as such, use of antimicrobial‐coated sutures should be considered.
Postoperative fibrin formation to create a complete seal of a surgical wound takes 3–5 hours and therefore protection of the fresh surgical wound from its environment is recommended [79]. Considering evidence for environmental contamination from the anesthesia transportation gurney and radiology table used for postoperative radiographs, immediate protection with a sterile dressing is recommended [46]. Despite these recommendations, direct comparison of a topical adhesive applied to the incision, an adhesive antimicrobial barrier dressing, and no dressing following TPLO surgery found no significant difference in SSI rates amongst the dressing options [28]. In another study involving significant protocol changes for animals undergoing TPLO surgery, the addition of a soft padded bandage with mupirocin ointment directly on the surgical wound, use of single‐use gloves for all postoperative patient interactions, and placement of an Elizabethan collar for additional barrier protection resulted in an 88% reduction in the odds ratio for developing an SSI [64]. While many factors likely contributed to this SSI reduction, simple incisional protective measures are easy to implement into routine postoperative care. Additionally, while the use of gloves does not replace appropriate hand hygiene, sterile gloves are recommended to be worn during examination of clean surgical wounds to prevent transmission of bacteria to the surgical site [80].
The overall goal of antimicrobial therapy in orthopedic surgery is to reduce the bacterial load present at the time of surgery. The bacteria of concern are skin commensals, most commonly Staphylococcus spp., and are most susceptible to cefazolin. The administration of antimicrobials for this purpose is required only during the period of risk (i.e., the surgical procedure) and is not required preoperatively and rarely required postoperatively.
Читать дальше