1 ...6 7 8 10 11 12 ...25 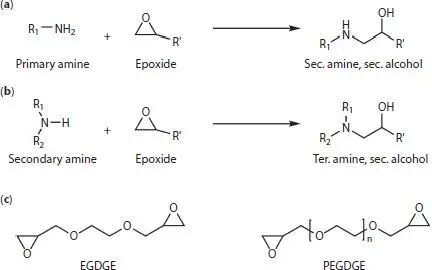
Figure 1.6 Reactions of (a) primary and (b) secondary amines with epoxides. (c) Main epoxide-based cross-linkers used for enzyme immobilization. R 1and R 2refer to groups in the enzyme or polymer, while R’ refers to groups in the cross-linker.
Different immobilization techniques can be coupled together. For example, Christwardana et al . employed a two-step immobilization of laccase and GOx. First, they created layer-by-layer (LbL) assemblies of each enzyme and PEI on the surface of carbon nanotubes. Afterwards, the modified electrode was exposed to GA crosslinking the enzymes and (although not explicitly stated by them) probably the PEI as well. The cell containing an enzymatic biocathode including the GA cross-linking step showed a higher maximum power density compared to the one fabricated just by LbL. This increase in performance was, however, a modest 3.6% [53].
1.3.2 Preparation of Microbial Bioelectrodes
Preparation of microbial bioelectrodes starts with the selection of an inoculum source; electroactive inoculum sources, ecological role (synergistic relationships), metabolic pathways involved in extracellular electron transfer and production of value-added metabolites are some topics addressed via microbial bioelectrocatalysis.
The preparation of microbial electrodes requires the use of electroactive microorganisms, which are found in different natural and artificial niches [67]. Among the natural niches are marine sediments and different types of soils [68]. Artificial sources of electroactive microorganisms are urban and industrial wastewater [69], and compost and sludge from treatment plants [70]. Another frequent source of inoculum is the effluent and biofilm from another operating microbial electrochemical cell [67]. Electroactive species have been tested in pure cultures, mainly of Geobacter spp. and Shewanella spp. [71]. These bacterial species have been studied in depth because they show the two principal electron transfer mechanisms, direct and mediated electron transfer. A direct transfer mechanism is attributed to Geobacter spp. [72, 73] and a mixed but predominantly mediated mechanism is present in Shewanella spp. [74, 75]. The preparation of an inoculum may include mixed cultures of at least two electroactive species, and mixed cultures of electroactive with no electroactive species. Pure cultures of Geobacter sulfurreducens and Shewanella oneidensis were compared to a defined mixed culture of both of these microorganisms. The performance of Geobacter was ten times superior, and this observation was attributed to the biofilm thickness; on the contrary, Shewanella formed an unstable biofilm. The mixed culture improved the global performance by 38% which was associated to the increase in biofilm thickness and the planktonic growth of Shewanella rather than their incorporation to biofilm, since Shewanella sp. produces mediator-like metabolites [76]. In another investigation, S. oneidensis MR-1 was added to the Dehalococcoides- containing culture for dechlorination of trichloroethylene [77], while G. sulfurreducens was growth with Pseudomonas aeruginosa to investigate the interspecies electron transfer via an omics approach [78]. Anaerobic species such as Methanobacterium palustre have been used as inoculum for anodes, whereas cathodic biofilm has been composed of Clostridium , Desulfovibrio and Sporomusa species [35]. Unfortunately, there are very few studies with mixed cultures. This seems to be a wide field of research on microbial ecology of electroactive species, in both artificial and natural environments where electroactive and non-electroactive species coexist.
Microbial consortia have become widely used as biocatalysts due to the possibility of eliminating organic and inorganic contaminants, and to the robustness that the consortium provides to the bioelectrochemical process. The biocathode inoculation step has been performed with homoacetogenic microbial species ( Sporomusa ovata ) from anaerobic sludge; as well, salt marsh sediments have been tested as inoculum [79]. Typical inoculum sources are listed in Table 1.2.
Biofilm development on electrodes is of great importance for the direct electron transfer mechanism. Biofilm developing on solid surfaces presents a fluctuant nature which initiates with adhesion to the electrode, followed by a 2D propagation, and 3D thickening. Erosion or complete detachment is observed during biofilm aging [81]. In the biofilm formation, the microbial community varies as function of the environmental conditions; thus, although specific species are present in the inoculum, the final microbial community in biofilm may differ [82]. Evolution of the microbial community tends to enrichment of Geobacter spp. when the electrode is continuously polarized [83]. In addition to the inoculum source, the surface characteristics of the electrode affect the preparation of the bioelectrode. Carbonaceous materials pretreated with thermal and chemical methods favored the adhesion of bacillus- or coccus-shaped microorganisms as a function of the pretreatment. This qualitative observation was verified by the electrical current produced by each type of bioelectrode [84].
Table 1.2 Examples of microbial biocatalysts in the form of pure cultures and consortia [79, 80].
| Bioanode, pure culture |
Biocathode, pure culture |
| Geobacter sulfurreducens |
Chlorella vulgaris |
| Geobacter metallirreducens |
Sporomusa ovata |
| Shewanella putrefaciens |
Clostridium ljungdahlii |
| Shewanella oneidensis |
Sporomusa sphaeroides |
| Pseudomona aeruginosa |
Clostridium sp . |
| Desulfuromonas acetooxidans |
|
| Enterobacter cloacae |
|
| Aeomonas hydrophila |
|
| Bioanode, consortium |
Biocathode, consortium |
| Pulp mill wastewater |
Brewery waste |
| Domestic wastewater |
Activated sludge |
| Primary clarifier effluent |
Enriched homoacetogenic culture |
| Waste activated sludge |
Culture from previous microbial electrochemical system |
| Compost |
|
| Compost leachate |
|
1.4 Supports for Immobilization of Enzymes and Microorganisms for Biofuel Cells
The development of biofuel cells, BFCs (enzymatic fuel cells and microbial fuel cells) are of great interest as was mentioned above. The main limitations of these devices are their short lifetimes and low-power density associated with different losses ( Figure 1.7). These limitations are related to the issues raised during immobilization of biocatalysts on electrodes to enable a direct electron transfer. At present, many reported BFCs employ redox mediators as electron shuttles involving the addition of an extra interface between the fuel and the solid electrode. Moreover, mediators are typically expensive, unstable and potentially toxic [85].
Therefore, one of the efforts to increase the life-time and power density is related to the development of nanomaterials, which facilities the electron transfer between the enzymes/microbes and the electrode [86–89]. For this purpose, the morphological and electronic characteristics of nanomaterials must decrease the different losses displayed in Figure 1.7a. At the same time, the nature of the nanomaterial must be smartly selected since biocompatibility and toxicity are highly important in microbial fuel cells; antibacterial materials can cause losses of activity and durability. According to the science of materials, the modifications that can be done to a support can be classified as morphological or electronic ( Figure 1.8). The most commonly reported supports for BFCs are carbon allotropes because they are nontoxic, abundant, cheap, and easy to prepare and to reproduce [90–92]. The effect of nanomaterials on the performance of BFCs is directly related to the changes in the affinity between the biocatalyst and the nanomaterial from a point of view of adsorption and chemical integration [85]. The increase in surface area boosts the contact points of the biocatalyst, improving the electron flow from the fuel to the electrode and the diffusion of mediators in the electrodes. There are several reviews dealing with the use of nanomaterials in BFCs [6–14, 90–98]; however, in the following pages the use of supports will be covered from a materials science point of view dealing with the latest findings (2017–to date).
Читать дальше













