In polymer entrapment ( Figure 1.4b), enzymes are physically trapped between the network of the polymer chains. Although some interactions between the enzymes and the polymer matrix might exist, they are not the main cause for enzymes to be fixed in place. Electropolymerization [43] and photopolymerization [44] in the presence of enzyme have been used to trap glucose oxidase in the polymer layer. An interesting approach has been the use of tetrabutylammonium bromide (TBAB)-modified Nafion, to immobilize alcohol dehydrogenase, aldehyde dehydrogenase and even nanotube-bound laccase [7, 9]. The exchange of the protons in the Nafion for hydrophobic alkyl ammonium ions reduces the acidity of the polymer environment and widens the channels to allow the diffusion of relatively large enzymes substrates and cofactors [45].
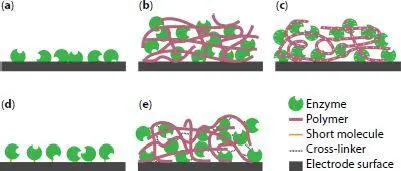
Figure 1.4 Schematic of popular enzyme immobilization techniques. (a) Adsorption (b) Polymer entrapment (c) Electrostatic entrapment (d) Covalent bonding and (e) Cross-linking.
Electrostatic entrapment ( Figure 1.4c) makes use of the charge in the amino acid residues of the redox enzyme. Depending on the relative values of the protein isoelectric point and environmental pH, enzymes can present a net positive or negative charge. Accordingly, they can be favorably attracted to ionic polymers bearing the opposite charge. Cationic polymers, such as poly(ethyleneimine)s (PEIs) [46], poly(allylamine) [47, 48] and chitosan [42, 49], have therefore been used to immobilize negatively charged enzymes through an anion exchange process. Conveniently, the two most commonly used enzymes, GOx and laccase, are negatively charged at their operating pH (usually 7–7.5 and 4.5–5.5, respectively [7, 50, 51]). This approach has been taken further to form a structure of alternating layers of enzyme and cationic polymer, generally known as layer-by-layer (LbL) deposition. Using this strategy on both anodic and cathodic graphite electrodes, Rengaraj et al . achieved maximum power densities of 103 μW cm −2[50]. Although stronger than simple adsorption, the forces involved in electrostatic entrapment are relatively weak. Therefore, enzyme loss is still a problem that prevents this strategy to be reliable at making durable electrodes.
Chemical bonding of the enzymes to the substrate is an alternative that provides stronger immobilization. The main forms in which it is usually implemented are referred to in literature as covalent bonding and cross-linking. Strictly speaking, however, both methods involve the formation of a covalent bond with the enzyme. In so-called covalent bonding ( Figure 1.4d), the functional groups on the enzyme surface react either with the electrode surface directly or with a short molecule that is anchored to the electrode surface. While providing stable enzyme/surface linkage, the main problem of this technique is that the presence of the reactive groups at the electrode surface often causes significant stress on the enzyme’s tertiary structure. Therefore, denaturation is a common problem of these electrodes [52].
Cross-linking is an immobilization technique that attempts to ameliorate the downsides of covalent bonding, while still forming a covalent bond to the enzyme. To this end, cross linking agents are added which react with the surface groups in the enzyme surface ( Figure 1.4e). Frequently, the amine groups present in the lysine residues have been targeted. Cross-linkers for this functional group have either aldehyde or epoxy terminated ends. Upon reaction with primary amines, aldehydes form imines ( Figure 1.5a), creating a covalent double bond between the enzyme and the cross-linker. One of the most common cross linkers, partly due to its low cost, is glutaraldehyde (GA) ( Figure 1.5c). This aliphatic cross linker is used, for example, to create bonds between the lysine residues in GOx, laccase and horseradish peroxidase [13, 53, 54]. While most authors have used the GA in solution [53, 54], some have preferred to expose the enzyme-modified electrode to a vapor of the cross-linker [13, 55, 56]. This method is more economical in that the deposition solution can be used for a longer period. Mixtures of enzymes and cross-linkers are reactive, making these solutions unstable. In fact, some precipitates start appearing within minutes in some solutions containing enzyme and cross-linker.
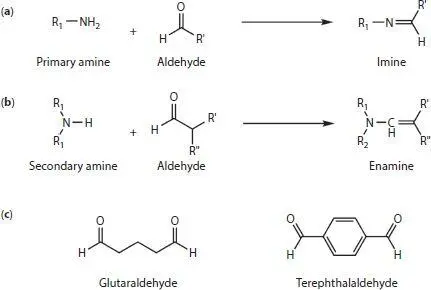
Figure 1.5 Reactions of (a) primary and (b) secondary amines with aldehydes. (c) Main aldehyde-based cross-linkers used for enzyme immobilization. R 1and R 2refer to groups in the enzyme or polymer, while R’ and R” refer to groups in the cross-linker.
Aldehydes can also react with the amine groups present in polymers such as poly(ethyleneimine)s (PEIs) [46]. Branched PEIs (BPEIs) ‡ possess primary, secondary and tertiary amines, while linear PEIs (LPEIs) possess almost exclusively secondary amines [57]. Therefore, when aldehydes react with LPEIs, they produce enamines instead ( Figure 1.5b). This cross-linking between enzymes and polymers is expected to strengthen the interaction compared to just using the electrostatic entrapment. Indeed, Chung et al . showed improved stability when cross-linking GOx to BPEI-covered carbon nanotubes using two aldehyde cross-linkers [58].
Kwon’s group has recently introduced the use of terephthalaldehyde ( Figure 1.5c) as a cross-linker for glucose oxidase and branched poly(ethyleneimine) [58]. They suggest that the conjugation between the C=N bonds and the phenyl group help improve the electron transfer across the whole composite. Furthermore, they tried different sequence of immobilization steps and found that, if the GOx is cross-linked first, it forms aggregates that can be later cross-linked to PEI-covered nanotubes. Using this methodology, they reported an additional 25% increase in their maximum power density compared to their previous approach [59].
Epoxide-based cross-linkers emerged as an alternative to aldehydes [60] driven partly by the reported toxicity of glutaraldehyde [61]. Epoxide groups are reactive to amine, hydroxyl and carboxyl functional groups [62]. When reacting with primary or secondary amines, they yield secondary or tertiary amines respectively and secondary alcohols ( Figures 1.6a, b). Polyethers containing epoxide ends have been the most popular epoxide cross-linkers in enzymatic fuel cells ( Figure 1.6c). Ethylene glycol diglycidyl ether (EGDGE), for example, has been the preferred cross-linker for the LPEI based hydrogels [54, 63–65]. Longer epoxide-terminated polyethers are generally known as poly (ethylene glycol) diglycidyl ether (PEGDGE) and are commercially available in a variety of chain lengths. These molecules have also been used to cross-link GOx in the presence of poly(Nvinylimidazole) [56] and poly(allylamine) [66] derivatives. A number of studies have been carried out to explore the effect of the amount of cross-linker in the electrode performance. Hickey et al . reported that the apparent Michaelis–Menten constant 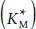 of GOx remained relatively non-affected by the amount of cross-linker (EGDGE), revealing that the affinity of the enzyme for its substrate was consistent regardless of the immobilization. Regarding the electrode current dependence on cross linker concentration, both EGDGE- and PEGDGE-based electrodes show the same trend; an initial increase at low concentrations goes through a maximum and decreases at higher concentrations [56, 62, 64]. The initial increase is believed to be caused by improved retention of the enzyme. After the optimal point, the current decreases, presumably due to decreased substrate mass transfer in the film [56].
of GOx remained relatively non-affected by the amount of cross-linker (EGDGE), revealing that the affinity of the enzyme for its substrate was consistent regardless of the immobilization. Regarding the electrode current dependence on cross linker concentration, both EGDGE- and PEGDGE-based electrodes show the same trend; an initial increase at low concentrations goes through a maximum and decreases at higher concentrations [56, 62, 64]. The initial increase is believed to be caused by improved retention of the enzyme. After the optimal point, the current decreases, presumably due to decreased substrate mass transfer in the film [56].
Читать дальше



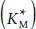 of GOx remained relatively non-affected by the amount of cross-linker (EGDGE), revealing that the affinity of the enzyme for its substrate was consistent regardless of the immobilization. Regarding the electrode current dependence on cross linker concentration, both EGDGE- and PEGDGE-based electrodes show the same trend; an initial increase at low concentrations goes through a maximum and decreases at higher concentrations [56, 62, 64]. The initial increase is believed to be caused by improved retention of the enzyme. After the optimal point, the current decreases, presumably due to decreased substrate mass transfer in the film [56].
of GOx remained relatively non-affected by the amount of cross-linker (EGDGE), revealing that the affinity of the enzyme for its substrate was consistent regardless of the immobilization. Regarding the electrode current dependence on cross linker concentration, both EGDGE- and PEGDGE-based electrodes show the same trend; an initial increase at low concentrations goes through a maximum and decreases at higher concentrations [56, 62, 64]. The initial increase is believed to be caused by improved retention of the enzyme. After the optimal point, the current decreases, presumably due to decreased substrate mass transfer in the film [56].










