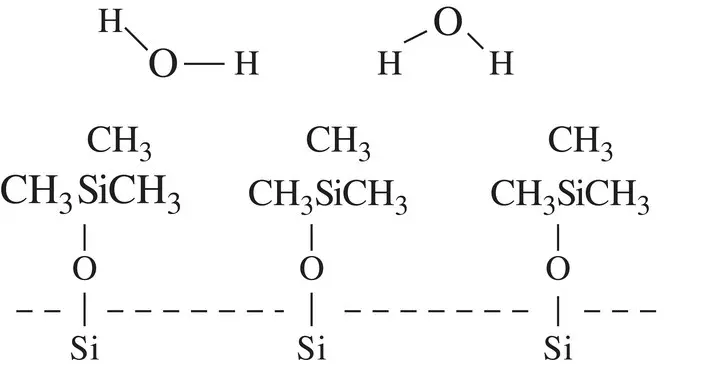
Figure 1.4Water molecules can only weakly interact (by vdw forces) with a methylated glass surface.
WETTING PROPERTIES AND THEIR INDUSTRIAL IMPORTANCE
These methylated groups cannot hydrogen bond, and hence water now does not wet and instead forms beads of high ‘contact angle’ ( θ ) droplets, and the glass now appears to be hydrophobic, with water droplets similar to those observed on paraffin wax.
This dramatic macroscopic difference in wetting behaviour is caused by only a thin molecular layer on the surface of glass and clearly demonstrates the importance of surface properties. The same type of effect occurs every day, when dirty fingers transfer natural grease and fats on to a drinking glass! Thorough cleaning of a glass flask (e.g., by washing in concentrated NaOH solution, as used in dishwashers) initially allows only a thin water film to coat (wet) the inside of the flask, producing visible coloured interference patterns (see photo). When contaminants from the air atmosphere slowly leak in via the stopper, this wetting film is displaced by a fine layer of non‐wetting droplets, forming an opaque mist film.
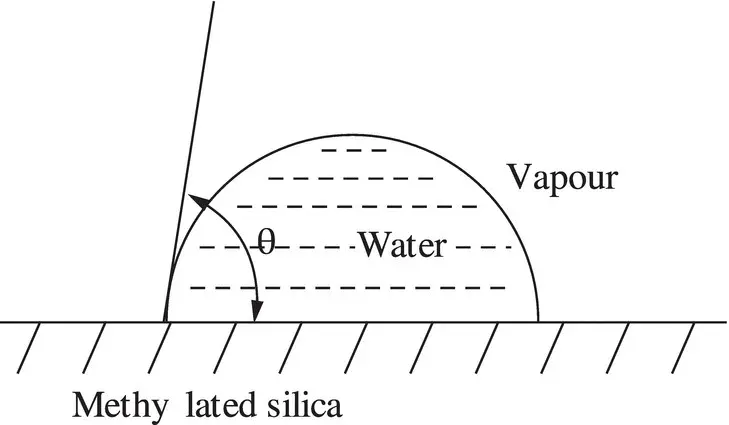
Figure 1.5A non‐wetting water droplet on the surface of methylated, hydrophobic silica.

Figure 1.6Clean glass flask with water wetting film and start of mist layer intrusion.
Surface treatments offer a remarkably efficient method for the control of macroscopic properties of materials. When insecticides are sprayed onto plant leaves, it is vital that the liquid wet and spread over the surface, rather than form a mist layer. Another important example is the froth flotation technique, used by industry to separate about a billion tons of ore each year. Whether valuable mineral particles will attach to rising bubbles and be ‘collected’ in the flotation process is determined entirely by the surface properties or surface chemistry of the mineral particle, and this can be controlled by the use of low levels of ‘surface‐active’ materials, which will selectively adsorb and change the surface properties of the mineral particles. Very large quantities of minerals are separated simply by the adjustment of their surface properties.
Although it is relatively easy to understand why some of the macroscopic properties of liquids, especially their shape, can depend on surface properties, it is not so obvious for solids. However, the strength of a solid is determined by the ease with which micro‐cracks propagate when placed under stress, and this depends on its surface energy. This understanding is crucial for the safe design of aircraft. That is, the design must consider the amount of (surface) work required to continue a crack and hence expose new surface. This also has the direct effect that materials are stronger in a vacuum, where their surface energy is not reduced by the adsorption of either gases or liquids typically available under atmospheric conditions. Much lighter structures can be made in space.
Many other industrial examples where colloid and surface chemistry plays a significant role will be discussed later; these include:
latex paint technology
water treatment
cavitation
emulsions and microemulsions
soil science
soaps and detergents
food science
mineral processing
Recommended Resource Books:
| Adamson, A. W. (1990) |
Physical Chemistry of Surfaces , 5th edn, New York, Wiley. |
| Birdi, K. S. (ed,) (1997) |
CRC Handbook of Surface and Colloid Chemistry , Boca Raton, FL: CRC Press. |
| Evans, D. F. and H. Wennerstrom (1999) |
The Colloidal Domain , 2nd edn, New York: Wiley. |
| Hiemenz, P.C (1997) |
Principles of Colloid and Surface Chemistry , 3rd edn, New York: Marcel Dekker. |
| Hunter, R. J. (1987) |
Foundations of Colloid Science , Vol. 1, Oxford: Clarendon Press. |
| Hunter, R. J. (1993) |
Introduction to Modern Colloid Science , Oxford: Oxford Sci. Publ. |
| Israelachvili, J, N. (1985) |
Intermolecular and Surface Forces , London: Academic Press. |
| Ninham, B. W. and P. Lo Nostro (2010) |
Molecular Forces and Self Assembly , Cambridge: Cambridge University Press. |
| Shaw, D. J. (1992) |
Introduction to Colloid and Surface Chemistry , 4th edn, Oxford, Boston: Butterworth‐Heinemann |
Appendix
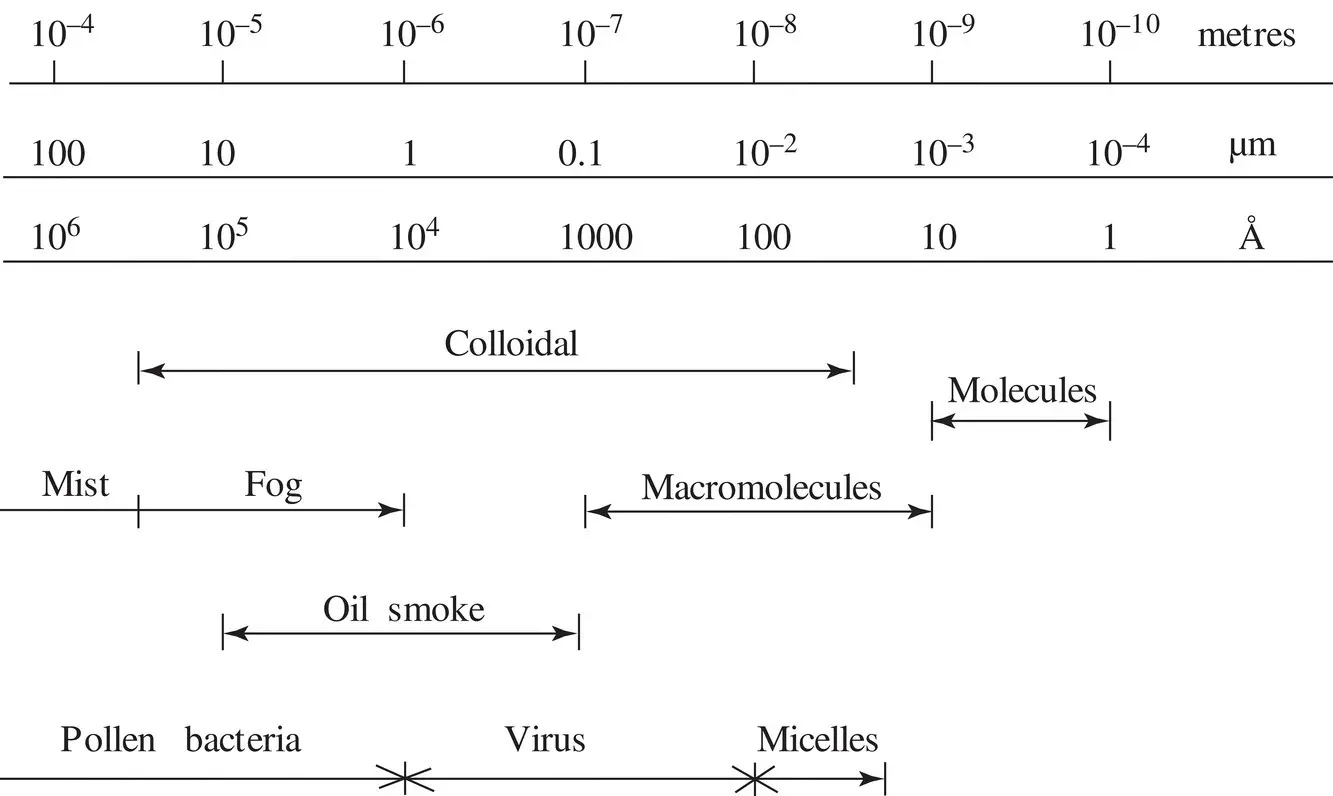
A. DISPERSED PARTICLE SIZES
B. SOME HISTORICAL NOTES ON COLLOID AND SURFACE CHEMISTRY
John Freindat Oxford (1675–1728) was the first person to realise that intermolecular forces are of shorter range than gravity.
Young(1805) estimated range of intermolecular forces at about 0.2 nm. Turns out to be something of an underestimate.
Youngand Laplace(1805) derived meniscus curvature equation.
Brown(1827) observed the motion of fine particles in water.
Johannes D. van der Waals(1837–1923) was a schoolmaster who produced a doctoral thesis on the effects of intermolecular forces on the properties of gases (1873).
Michael Faraday(1857) made colloidal solutions of gold.
Thomas Graham(1860) recognised the existence of colloids in the mid‐19th century.
Schulzeand Hardy(1882–1900) studied the effects of electrolytes on colloid stability.
Perrin(1903) used the terms ‘lyophobic’ and ‘lyophilic’ to denote irreversible and reversible coagulation.
Ostwald(1907) developed the concepts of ‘disperse phase’ and ‘dispersion medium’.
Gouyand Chapman(1910–1913) both independently used the Poisson‐Boltzmann equations to describe the diffuse electrical double‐ layer formed at the interface between a charged surface and an aqueous solution.
Ellis and Powis(1912–1915) introduced the concept of the critical zeta potential for the coagulation of colloidal solutions.
London(1920) first developed a theoretical basis for the origin of intermolecular forces.
Debye(1920) used polarisability of molecules to estimate attractive forces.
Debyeand Huckel(1923) used a similar approach to Gouy and Chapman to calculate the activity coefficients of electrolytes.
Stern(1924) introduced the concept of specific ion adsorption at surfaces.
Kallmannand Willstatter(1932) calculated van der Waals force between colloidal particles using summation procedure and suggested that a complete picture of colloid stability could be obtained on the basis of electrostatic double‐layer and van der Waals forces.
Читать дальше
















