Our understanding of these forces has led to our ability to selectively control the electrostatic repulsion and so create a powerful mechanism for controlling the properties of colloidal solutions. As an example, if we have a valuable mineral imbedded in a quartz rock, grinding the rock will separate out both pure individual quartz and the mineral particles, which can both be dispersed in water. The valuable mineral can then be selectively coagulated, whilst leaving the unwanted quartz in solution. This process is used widely in the mining industry as the first stage of mineral separation. The alternative of chemical processing, for example, by dissolving the quartz in hydrofluoric acid, would be both expensive and environmentally unfriendly.

Figure 1.1Scanning electron microscope image of dried mono‐disperse silica colloids.
It should be realised, at the outset, that colloidal solutions (unlike true solutions) will almost always be in a metastable state. That is, an electrostatic repulsion prevents the particles from combining into their most thermodynamically stable state of aggregation into the macroscopic form, from which the colloidal dispersion was (artificially) created in the first place. On drying, colloidal particles will often remain separated by these repulsive forces, as illustrated by the scanning electron microscope picture of mono‐disperse silica colloids.
TYPES OF COLLOIDAL SYSTEMS
The term ‘colloid’ usually refers to particles within the approximate size range of 50 Å to 50 μm, but this, of course, is somewhat arbitrary. For example, blood could be considered as a colloidal solution in which large blood cells are dispersed in water. They are stabilized by their negative charge, and so oppositely charged ions, for example, those produced by an alum stick, will coagulate the cells and hence stop bleeding. Often we are interested in solid dispersions in aqueous solutions, but many other situations are also of interest and industrial importance. Some examples are given in the following table.
The properties of colloidal dispersions are intimately linked to the high surface area of the dispersed phase and the chemistry of these interfaces. This linkage is well illustrated by the titles of two of the main journals in this area: the Journal of Colloid and Interface Science and Colloids and Surfaces . The natural combination of colloid and surface chemistry represents a major area of both research activity and industrial development. It has been estimated that something like 20 per cent of all chemists in industry work in this area. The more recent term nano is also applied to these small scale materials, because of their typical nanometre size.
Table 1.1
| Dispersed phase |
Dispersion medium |
Name |
Examples |
| Liquid |
Gas |
Liquid aerosol |
Fogs, sprays |
| Solid |
Gas |
Solid aerosol |
Smoke, dust |
| Gas |
Liquid |
Foam |
Foams |
| Liquid |
Liquid |
Emulsion |
Milk, mayonnaise |
| Solid |
Liquid |
‘Sol’, |
Au sol, AgI sol. |
|
|
Paste at high |
Toothpaste |
|
|
concentration |
|
| Gas |
Solid |
Solid foam |
Expanded polystyrene |
| Liquid |
Solid |
Solid emulsion |
Opal, pearl |
| Solid |
Solid |
Solid suspension |
Pigmented plastics |
THE LINK BETWEEN COLLOIDS AND SURFACES
The link between colloids and surfaces follows naturally from the fact that particulate matter has a high surface area to mass ratio. The surface area of a 1 cm diameter sphere (4π r 2) is 3.14 cm 2, whereas the surface area of the same amount of material but in the form of 0.1 micron diameter spheres (i.e., the size of the particles in latex paint) is 314,000 cm 2. The enormous difference in surface area is one of the reasons why the properties of the surface become very important for colloidal solutions. One everyday example is that organic dye molecules or pollutants can be effectively removed from water by adsorption onto particulate or granular activated charcoal because of its high surface area. This process is widely used for water purification and in the oral treatment of poison victims.
Although it is easy to see that surface properties will determine the stability of colloidal dispersions, it is not so obvious why this can also be the case for some properties of macroscopic objects. As one important illustration, consider the interface between a liquid and its vapour:
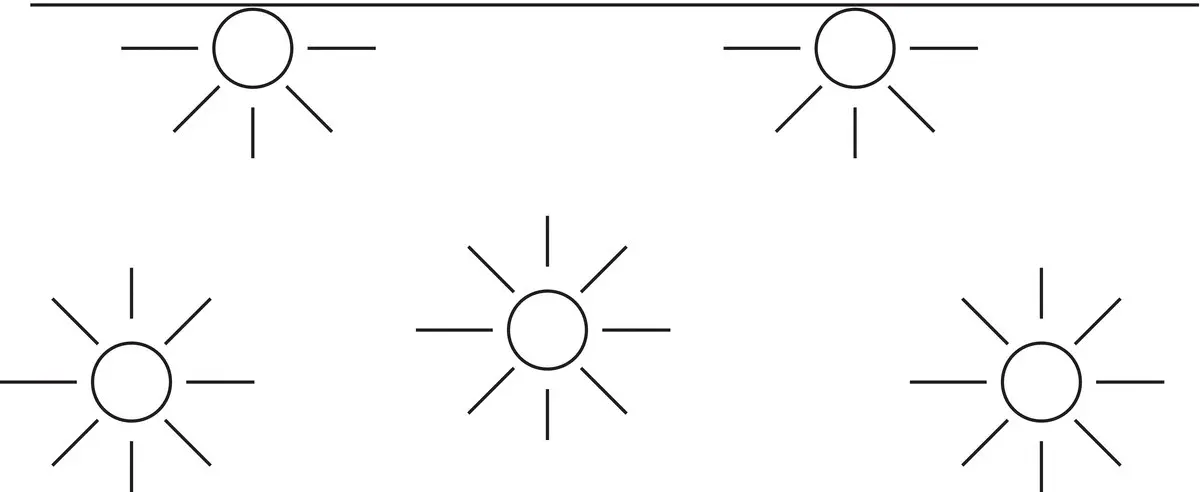
Figure 1.2Schematic diagram to illustrate the complete bonding of liquid molecules in the bulk phase but not at the surface.
Table 1.2
| Liquid |
Surface Energy in mJm −2(at 20 °C) |
Type of Intermolecular Bonding |
| Mercury |
485 |
metallic |
| Water |
72.8 |
hydrogen bonding + vdw |
| n ‐Octanol |
27.5 |
hydrogen bonding + vdw |
| n ‐Hexane |
18.4 |
vdw |
| Perfluoro‐octane |
12 |
weak vdw |
Molecules in the bulk of the liquid can interact via attractive forces (e.g., van der Waals) with a larger number of nearest neighbours than those at the surface. The molecules at the surface must therefore have a higher energy than those in bulk, since they are partially freed from bonding with neighbouring molecules. Thus, work must be done to take fully interacting molecules from the bulk of the liquid to create a new surface. This work gives rise to the surface energy or tension of a liquid. Hence, the stronger the intermolecular forces between the liquid molecules, the greater this work will be, as is illustrated in the table.
The influence of this surface energy can also be clearly seen on the macroscopic shape of liquid droplets, which in the absence of all other forces will always form a shape of minimum surface area – that is, a sphere in a gravity‐free system. This is the reason why small mercury droplets are always spherical. Note that the term ‘interface’ is often used where the surface is formed between two different materials.
Although a liquid will always try to form a minimum surface area shape, if no other forces are involved, it can also interact with other macroscopic objects to reduce its surface tension via molecular bonding to another material, such as a suitable solid. Indeed, it may be energetically favorable for the liquid to interact and ‘wet’ another material. The wetting properties of a liquid on a particular solid are very important in many everyday activities and are determined solely by surface properties, which are derived from intermolecular forces. One important and common example is that of the behaviour of water on clean glass. Water wets clean glass because of the favourable hydrogen bond interaction between the surface silanol groups on glass and adjacent water molecules, as illustrated below.
However, exposure of glass to Me 3SiCl vapour rapidly produces a 0.5 nm layer of methyl groups on the surface:
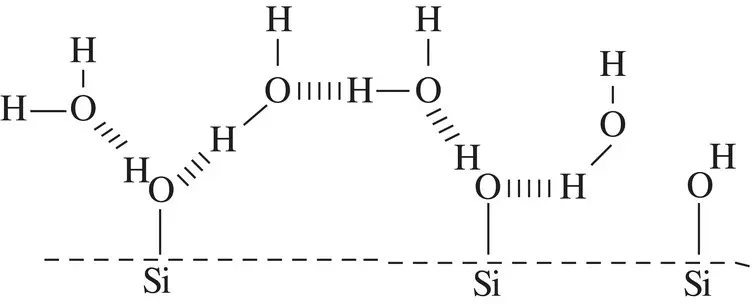
Figure 1.3Water molecules form hydrogen bonds with the silanol groups at the surface of clean glass.
Читать дальше















