The book contains some recommended experiments, which we have found to work well and stimulate students to consider both the fundamental theory and industrial applications. Sample questions have also been included in some sections, with detailed answers available on our web site.
Although the text has been primarily aimed at students, researchers in cognate areas may also find some of the topics stimulating. A reasonable background in chemistry or physics is all that is required.
We also would like to gratefully acknowledge important contributions from several students, including John Antony and Mojtaba Taseidifar (for Chapter 3) and Mathew Francis and Rui Wei (for Chapter 11).
Richard M. Pashley
Marilyn E. Karaman
October 2020
About the Companion Website
This book is accompanied by a companion website.
www.wiley.com/go/pashley/appliedcolloid2e 
This website includes:
Powerpoint slides of Figures from the book
Introduction to the nature of colloids and the linkage between colloids and surface properties. The importance of size and surface area. Introduction to wetting and the industrial importance of particle size and surface modifications .
INTRODUCTION TO THE NATURE OF COLLOIDAL SOLUTIONS
The difference between macroscopic and microscopic objects is clear from everyday experience. For example, a glass marble will sink rapidly in water; however, if we grind it into sub‐micron‐sized particles, these will float or disperse freely in water, producing a visibly cloudy ‘solution’, which can remain stable for hours or days. In this process we have, in fact, produced a ‘colloidal’ dispersion or solution. This dispersion of one (finely divided or microscopic) phase in another is quite different to the molecular mixtures or ‘true’ solutions formed when we dissolve ethanol or common salt in water. Microscopic particles of one phase dispersed in another are generally called colloidal solutions or dispersions. Both nature and industry have found many uses for this type of solution. We will see later that the properties of colloidal solutions are intimately linked to the high surface area of the dispersed phase, as well as to the chemical nature of the particle’s surface.
Historical note: The term ‘colloid’ is derived from the Greek word for glue, ‘kolla’. It was originally used for gelatinous polymer colloids, which were identified by Thomas Graham in 1860 in experiments on osmosis and diffusion .
It turns out to be very useful to dissolve (or more strictly disperse) solids, such as minerals and metals, in water. But how does it happen? We can see why from simple physics. Three fundamental forces operate on fine particles in solution:
1 A gravitational force, tending to settle or raise particles depending on their density relative to the solvent.
2 A viscous drag force, which arises as a resistance to motion, since the fluid has to be forced apart as the particle moves through it.
3 The ‘natural’ kinetic energy of particles and molecules, which causes random Brownian motion.
If we consider the first two non‐random forces, we can easily calculate the terminal or limiting velocity, V , (for settling or rising, depending on the particle’s density relative to, say, water) of a spherical particle of radius r . Under these conditions, the viscous drag force must equal the gravitational force. Thus, at a settling velocity, V , the viscous drag force is given by: F drag= 6π rVη = 4π r 3 g ( ρ p− ρ w)/3 = F gravity, the gravitational force, where η is the viscosity of water and the density difference between particle and water is ( ρ p− ρ w). Hence, if we assume a particle‐water density difference of +1 g cm ‐3, we obtain the results:
| r /Å |
100 |
1000 |
10,000 |
10 5 |
10 6 |
| r /μm |
0.01 |
0.1 |
1 |
10 |
100 |
| V /cm/sec |
2 × 10 −8 |
2 × 10 −6 |
2 ×10 ‐4 |
2 × 10 −2 |
2 |
Clearly, from factors (1) and (2), small particles will take a very long time to settle, and so a fine dispersion will be stable almost indefinitely, even for materials denser than water. But what of factor (3)? Each particle, independent of size, will have a kinetic energy, on average, of around 1 kT . So the typical random speed ( ν ) of a particle (in any direction) will be roughly given by:
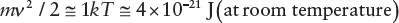
Again, if we assume that ρ p= 2 g cm −3, then we obtain the results:
| r /Å |
100 |
1000 |
10,000 |
10 5 |
10 6 |
| r /μm |
0.01 |
0.1 |
1 |
10 |
100 |
| ν /cm/sec |
10 2 |
3 |
0.1 |
3 × 10 −3 |
1 × 10 ‐4 |
These values suggest that kinetic random motion will dominate the behaviour of small particles, which will not settle, and the dispersion will be completely stable. However, this point is really the beginning of ‘colloid science’. Since these small particles have this kinetic energy they will, of course, collide with other particles in the dispersion, with collision energies ranging up to at least 10 kT (since there will actually be a distribution of kinetic energies). If there are attractive forces between the particles – as is reasonable since most colloids were initially formed via a vigorous mechanical process of disruption of a macroscopic or large body – each collision might cause the growth of larger aggregates, which will then, for the reasons already given, settle out and we will no longer have a stable dispersion! The colloidal solution will coagulate and produce a solid precipitate at the bottom of a clear solution.
There is, in fact, a ubiquitous force in nature, called the van der Waals (vdw) force, which is one of the main forces acting between molecules and is responsible for holding together many condensed phases, such as solid and liquid hydrocarbons and polymers. It is responsible for about one third of the attractive force holding liquid water molecules together. This force was actually first observed as a correction to the ideal gas equation and is attractive even between neutral gas molecules, such as oxygen and nitrogen, in a vacuum, which is why they can be liquified. Although electromagnetic in origin (as we will see later), it is much weaker than the coulombic force acting between ions, such as in salt crystals.
THE FORCES INVOLVED IN COLLOIDAL STABILITY
Although van der Waals forces will always act to coagulate dispersed colloids, it is possible to generate an opposing repulsive force of comparable strength. This force arises because most materials, when dispersed in water, ionise to some degree or selectively adsorb ions from the solution and hence become charged. Two similarly charged colloids will repel each other via an electrostatic repulsion, which will oppose coagulation. The stability of a colloidal solution is therefore critically dependent on the charge generated at the surface of the particles. The combination of these two forces, attractive van der Waals and repulsive electrostatic forces, forms the fundamental basis for our understanding of the behaviour and stability of colloidal solutions. The corresponding theory is referred to as the DLVO (after Derjaguin, Landau, Verwey and Overbeek) theory of colloid stability, which we will consider in greater detail later. The stability of any colloidal dispersion is thus determined by the behaviour of the surface of the particle via its surface charge and its short‐range attractive van der Waals force.
Читать дальше