References
1.Hashim PW, Nia JK, Taliercio M, Goldenberg G. Local anesthetics in cosmetic dermatology. Cutis 2017;99:393–397.
2.Dayan SH, Bassichis BA. Facial dermal fillers: Selection of appropriate products and techniques. Aesthetic Surg J 2008;28:335–347.
3.Smith KC, Comite SL, Balasubramanian S, Carver A, Liu JF. Vibration anesthesia: A noninvasive method of reducing discomfort prior to dermatologic procedures. Dermatol Online J 2004;10:1.
4.Zeiderman MR, Kelishadi SS, Tutela JP, et al. Vapocoolant anesthesia for cosmetic facial rejuvenation injections: A randomized, prospective, split-face trial. Eplasty 2018;18:e6.
5.Nestor MS, Ablon GR, Stillman, MA. The use of a contact cooling device to reduce pain and ecchymosis associated with dermal filler injections. J Clin Aesthet Dermatol 2010;3:29–34.
6.Mally P, Czyz CN, Chan NJ, Wulc AE. Vibration anesthesia for the reduction of pain with facial dermal filler injections. Aesth Plast Surg 2014;38:413–418.
7.Shapiro FE. Anesthesia for outpatient cosmetic surgery. Curr Opin Anaesthesiol 2008;21:704–710.
8.Sobanko JF, Miller CJ, Alster TS. Topical anesthetics for dermatologic procedures: A review. Dermatol Surg 2012;38:709–721.
9.Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol 2016;74:1201–1219.
10.Desai MS. Office-based anesthesia: New frontiers, better outcomes, and emphasis on safety. Curr Opin Anaesthesiol 2008;21:699–703.
11.Bogan V. Anesthesia and safety considerations for office-based cosmetic surgery practice. AANA J 2012;80:299–305.

3
Dermal Filler Selection
A cardinal rule in dentistry also applies to esthetic facial rejuvenation with fillers: Product selection is key to achieving successful results. Yet, there are dozens of filler products for practitioners to choose from, including some autologous agents. A survey of the dermal filler landscape, including a brief history of its evolution, will give the novice esthetic practitioner a bird’s-eye perspective of how the field developed, along with a ground-level understanding of how to quickly navigate the market and make informed choices.
Dermal Filler Classifications
Dermal fillers can be classified in terms of longevity, biodegradability, and mechanism of action.
Longevity
Facial dermal fillers are usually described as short-acting, long-acting, or “permanent,” but there is wide latitude in how these terms are defined. Generally speaking, a short-acting filler can be expected to last up to 1 year, a long-acting filler up to 2 years, and a filler labeled as permanent (an obvious misnomer) for 5 years or more. 1,2In most discussions, duration of effect of a given product is a relative concept that makes reference to other products as the standard of comparison.
Biodegradability
Biodegradability, which has a major impact on a product’s longevity, is another classification that strays into gray territory. 3,4A product or agent can be labeled as (1) a biodegradable filler, (2) a filler with biodegradable particles, or (3) a nondegradable filler. Biodegradable fillers are fully reabsorbed by the body and are consequently short-lived. Fillers with biodegradable particles act by stimulating a response in the body to produce its own collagen before resorbing, which results in a longer-lasting effect. Nondegradable fillers are dual-acting: They induce a foreign-body reaction that stimulates the deposition of new collagen, but the nondegradable particles ultimately become permanently integrated into the connective tissue of the skin.
Mechanism of action
As the discussion of biocompatibility suggests, fillers can also be classified according to their mechanism of action. 5,6In this category, a distinction is made between a volumizer , which is a filler that achieves its effect by taking up space (giving added importance to the amount of product injected), and a stimulator , one that in some way induces the body to produce new collagen. Again, some products fit neatly into one of these two categories, while others fall somewhere in between.
As with dental treatments, a one-size-fits-all approach to facial augmentation will not yield optimal results. As dermal filling options continue to expand and evolve, the clinician must understand the unique properties and characteristics of each product and apply that knowledge to developing an individual treatment plan for each patient. Box 3-1lists the qualities of an ideal filler product in terms of its safety, effectiveness, and convenience. 7–9The remainder of this chapter explains how each agent available is classified and measures up to the qualities of an ideal filler. For convenience, the discussion proceeds from short-acting to long-acting to permanent fillers, which—not coincidently—corresponds to the order in which they evolved.
BOX 3-1Properties of the ideal dermal filler
Safety
Nonimmunogenic
Noncarcinogenic
Nonteratogenic
Noninfectious
Efficacy
Long-term benefit
Natural feeling
Nonmigratory
Reproducible results
Practicality
Cost-effective
Easy to use
FDA-cleared
Removable or reversible
Long shelf life
As in dentistry, many products are used off-label by experienced dermatologists; the present discussion is restricted to those that have been cleared by the U.S. Food and Drug Administration (FDA) for specific facial cosmetic indications (Fig 3-1 and Table 3-1). 10
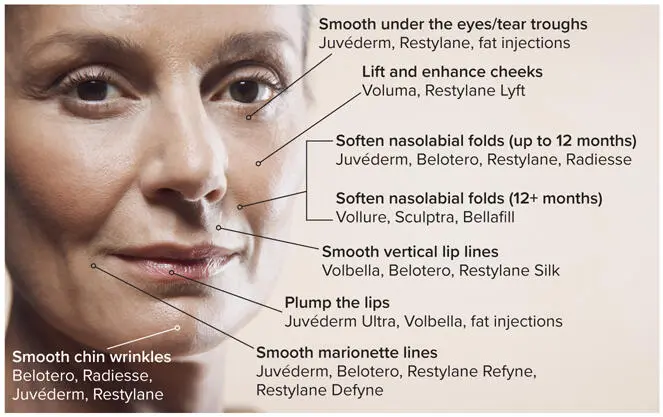
FIG 3-1Dermal filler products have been FDA cleared for specific treatments but are often used off-label in other areas of the face. (Adapted from the American Society of Plastic Surgeons. 10 )
TABLE 3-1Dermal fillers approved by the FDA for use in the United States
| Trade name (manufacturer) |
Year approved |
Approved uses |
| Hyaluronic acid |
| Restylane Lyft (Galderma) |
2018 |
Deep dermis to superficial subcutis injection for correction of moderate to severe facial folds and wrinkles; subcutaneous to supraperiosteal injection for cheek augmentation and midface deficiencies; subcutaneous injection for correction of volume deficit in the dorsal hand. |
| Restylane Refyne (Galderma) |
2016 |
Mid-to-deep dermis injection for correction of moderate to severe facial wrinkles and folds. |
| Restylane Defyne (Galderma) |
2016 |
Mid-to-deep dermis injection for correction of moderate to severe deep facial wrinkles and folds. |
| Restylane Silk (Galderma) |
2014 |
For lip augmentation and dermal implantation and for correction of perioral rhytids. |
| Restylane (Galderma) |
2003 |
Mid-to-deep dermis injection for correction of moderate to severe facial wrinkles and folds. |
| Revanesse Versa (Prollenium) |
2018 |
Mid-to-deep dermis injection for correction of moderate to severe facial wrinkles and folds. |
| Revanesse Versa Plus (Prollenium) |
2018 |
Mid-to-deep dermis injection for correction of moderate to severe facial wrinkles and folds (+ lidocaine). |
| Teosyal RHA 2 (Teoxane) |
2017 |
Mid-to-deep dermis injection for correction of moderate to severe dynamic facial wrinkles and folds. |
| Teosyal RHA 3 (Teoxane) |
2017 |
Mid-to-deep dermis injection for correction of moderate to severe dynamic facial wrinkles and folds. |
| Teosyal RHA 4 (Teoxane) |
2017 |
Deep dermis to superficial subcutaneous tissue injection for correction of moderate to severe dynamic facial wrinkles and folds. |
| Juvéderm Vollure XC (Allergan) |
2017 |
Mid-to-deep dermis injection for correction of moderate to severe facial wrinkles and folds. |
| Juvéderm Volbella XC (Allergan) |
2016 |
Injection into the lips for lip augmentation and for correction of perioral rhytids. |
| Juvéderm Voluma XC (Allergan) |
2013 |
Deep subcutaneous and/or supraperiosteal injection for cheek augmentation for correction of age-related volume deficit in the midface. |
| Juvéderm Ultra (Allergan) |
2006 |
Mid-to-deep dermis injection for correction of moderate to severe facial wrinkles and folds, and lip augmentation. |
| Juvéderm Ultra Plus (Allergan) |
2008 |
Mid-to-deep dermis injection for correction of moderate to severe facial wrinkles and folds. |
| Belotero Balance (Merz North America) |
2011 |
Injection into facial tissue to smooth wrinkles and folds, especially around the nose and mouth. |
| Poly-l-lactic acid (PLLA) |
| Sculptra Aesthetic (Galderma) |
2009 |
Injection into facial tissue for correction of shallow to deep nasolabial fold contour deficiencies and other facial wrinkles. |
| Calcium hydroxyapatite (CaHA) |
| Radiesse (Merz North America) |
2006 |
Subdermal injection for correction of moderate to severe facial wrinkles and folds. |
| Radiesse Plus (Merz North America) |
2006 |
Subdermal injection for correction of moderate to severe facial wrinkles and folds (+ lidocaine). |
| Polymethylmethacrylate (PMMA) microspheres |
| Bellafill (Suneva Medical) |
2006/2014 |
Injection into facial tissue for correction of moderate to severe facial wrinkles and folds. In 2014, it was also cleared for moderate to severe pitted atrophic facial acne scars. |
Short-Acting Fillers
Читать дальше














