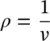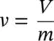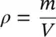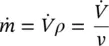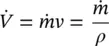1 ...6 7 8 10 11 12 ...27 Digital display thermometers: A wide range of digital display thermometers, for example, hand‐held battery‐powered displays and panel‐mounted mains or battery units, are available commercially. Displays can be provided for use with all standard thermocouples or platinum resistance thermometers with several digits and 0.1°C resolution. Table 1.1 Some of the most common thermocouples.TypeCommon namesTemperature range (°C)TCopper–constantan (C/C)−250 to 400JIron–constantan (I/C)−200 to 850ENickel chromium–constantan or chromel–constantan−200 to 850KNickel chromium–nickel aluminum or chromel–alumel (C/A)−180 to 1100—Nickel 18% molybdenum–nickel0 to 1300NNicrosil–nisil0 to 1300SPlatinum 10% rhodium–platinum0 to 1500RPlatinum 13% rhodium–platinum0 to 1500BPlatinum 30% rhodium–platinum 6% rhodium0 to 1600
It is important to emphasize that before temperature can be controlled, it must be sensed and measured accurately. There are several potential sources of error for temperature measurement devices, including sensor properties, contamination effects, lead lengths, immersion, heat transfer, and controller interfacing. In temperature control, there are many sources of error that can be minimized by careful consideration of the type of sensor, its working environment, the sheath or housing, extension leads, and the instrumentation. An awareness of potential errors is vital in many applications dealt within this book. Selection of temperature measurement devices is a complex task and has been discussed only briefly here. It is important to remember the following: “choose the right tool for the right job.”
1.3.4 Specific Volume and Density
The specific volume v is the volume per unit mass of a substance, usually expressed in cubic meters per kilogram (m 3/kg) in the SI system and in cubic feet per pound (ft 3/lb) in the English system. The density ρ of a substance is defined as the mass per unit volume, and is therefore the inverse of the specific volume:
(1.5) 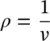
The units of density are kg/m 3in the SI system and lb/ft 3in the English system. Specific volume is also defined as the volume per unit mass, and density as the mass per unit volume, that is,
(1.6) 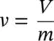
and
(1.7) 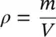
Both specific volume and density are intensive properties and are affected by temperature and pressure.
1.3.5 Mass and Volumetric Flow Rates
Mass flow rate is defined as the mass flowing per unit time (kg/s in the SI system and lb m/s in the English system). Volumetric flow rates are given in m 3/s in the SI system and ft 3/s in the English system. The following expressions can be written for the flow rates in terms of mass, specific volume, and density:
(1.8) 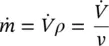
and
(1.9) 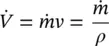
1.4 General Aspects of Thermodynamics
In this section, we briefly introduce some general aspects of thermodynamics that are related to energy storage systems and applications.
1.4.1 Thermodynamic Systems
A thermodynamic system is a device or combination of devices that contains a certain quantity of matter. It is important to carefully define a system under consideration and its boundaries. We can define three important types of systems as follows:
Closed system: This is defined as a system across the boundaries of which no material crosses. It, therefore, contains a fixed quantity of matter. In some books, it is also called a control mass.
Open system: This is defined as a system in which material (mass) is allowed to cross its boundaries. The term open system is also called a control volume.
Isolated system: This is a closed system that is not affected by the surroundings. No mass, heat, or work crosses its boundary.
A process is a physical or chemical change in the properties of matter or the conversion of energy from one form to another. In some processes, one property remains constant. The prefix “iso” is employed to describe such a process, for example, isothermal (constant temperature), isobaric (constant pressure), and isochoric (constant volume).
A cycle is a series of thermodynamic processes in which the end‐point conditions or properties of the matter are identical to the initial conditions.
1.4.4 Thermodynamic Property
This is a physical characteristic of a substance, which is used to describe its state. Any two properties usually define the state or condition of a substance, from which all other properties can be derived. Some examples are temperature, pressure, enthalpy, and entropy. Thermodynamic properties are classified as intensive properties (independent of the mass, e.g. pressure, temperature, and density) and extensive properties (dependent on the mass, e.g. mass and total volume). Extensive properties on a per unit mass basis, such as specific volume, become intensive properties. Property diagrams of substances can be presented in graphical form to summarize the main properties listed in property tables, for example, refrigerant tables.
1.4.5 Sensible and Latent Heats
It is known that all substances can hold a certain amount of heat; this property is their thermal capacity. When a liquid is heated, its temperature rises to the boiling point. This is the highest temperature that the liquid can reach at the measured pressure. The heat absorbed by the liquid in raising the temperature to the boiling point is called sensible heat . The heat required to convert the liquid to vapor at the same temperature and pressure is called latent heat . This is the change in enthalpy during a state change (the amount of heat absorbed or rejected at constant temperature at any pressure, or the difference in enthalpies of a pure condensable fluid between its dry saturated state and its saturated liquid state at the same pressure).
1.4.6 Latent Heat of Fusion
Fusion is associated with the melting and freezing of a material. For most pure substances, there is a specific melting/freezing temperature, relatively independent of the pressure. For example, ice begins to melt at 0°C. The amount of heat required to melt one kilogram of ice at 0°C to one kilogram of water at 0°C is called the latent heat of fusion of water, and equals 334.92 kJ/kg. The removal of the same amount of heat from one kilogram of water at 0°C changes it back to ice.
A vapor is a gas at or near equilibrium with the liquid phase – a gas under the saturation curve or only slightly beyond the saturated vapor line. Vapor quality is theoretically assumed; that is, when vapor leaves the surface of a liquid, it is pure and saturated at the particular temperature and pressure. In actuality, tiny liquid droplets escape with the vapor. When a mixture of liquid and vapor exists, the ratio of the mass of the liquid to the total mass of the liquid and vapor mixture is called the quality , and is expressed as a percentage or decimal fraction. Superheated vapor is the saturated vapor to which additional heat has been added, raising the temperature above the boiling point. Let us consider a mass m with a quality x . The volume is the sum of the volumes of both the liquid and the vapor, as defined below:
Читать дальше