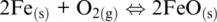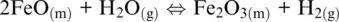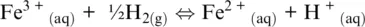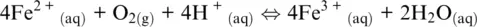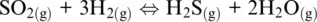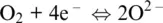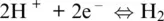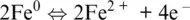Roberto Moretti1,2 and Daniel R. Neuville1
1 Université de Paris, Institut de Physique du Globe de Paris, Paris, France
2 Observatoire Volcanologique et Sismologique de Guadeloupe, Institut de Physique du Globe de Paris, Gourbeyre, France
The basic aspects of redox geochemistry are reviewed to provide a useful compendium of the redox connection between the aqueous‐hydrothermal and igneous realms of Earth. The redox description of a system is intimately coupled to the knowledge of acid‐base properties of the solvent in which redox exchanges take place. For magmas, and then silicate melts, approaches reporting the redox state were so far cantered around the sole concept of oxygen fugacity, f O 2. Mastering the concept of f O 2in experimental and observational petrology was the key to constrain the processes behind the very large range of relative oxygen fugacity observed on Earth. Although current descriptions of silicate melts and magma thermodynamic properties are mainly based on oxides or mineral-like molecular components, disregarding the actual melt reactivity poses many limits in our understanding of the true chemical exchanges involving oxygen, iron and the other redox-sensitive elements. Because silicate melts, unlike aqueous solutions, lack of a full acid-base description, compositional dependencies are solved by means of empirical treatments based on oxides and their combinations. However, these can bias the interpretation of redox exchanges recorded in analyzed samples and used to identify the several processes (e.g., batch or fractional crystallization, elemental recycling, degassing, deep fluid infiltration) which characterize magma evolution and its geodynamic environment. This short compendium aims at stimulating the quest for a comprehensive and unifying picture of the acid-base and redox properties of melts from which we could extrinsic its reactivity in way similar to aqueous solutions and molten salts.
1.1. GENERAL ASPECTS AND RATIONALE
1.1.1. Oxidation Number, Electron Transfer, and Half‐Reactions
Oxidation‐reduction (redox) geochemistry studies those natural reactions occurring on Earth in which the transfer of electrons determines a change in the oxidation number of participating chemical species. Oxidation state (or oxidation number) measures the degree of oxidation of an atom in a substance and it is the (hypothetical) charge of an atom if all bonds to atoms of different elements were 100% ionic, with no covalent bond fraction. The oxidation state of an atom is indicated with Roman numerals, whereas Arabic numbers are used for the charge on compound (e.g., in SiO 4 4–, silicon has oxidation number IV and oxygen has –II, whereas 4– is the formal charge of the whole silicate ion). Charge and oxidation number are the same for monoatomic ions. The oxidation number can be positive, negative, or zero, and it can have a fractional value as well, and although very similar, it does not correspond to valency, which is an atom property and represents the number of bonds the atom needs to become stable, i.e., to complete either duplet or octet rule.
Many redox reactions are familiar to us, such as fire and combustion, rusting, and dissolution of metals. Transition metals and main group elements (e.g., N, halogens, O, S, C) have multiple oxidation states and important redox chemistry, which affect element distribution within the geochemical shells on Earth but also through the boundaries between such shells (e.g., Moretti et al., 2020a). For instance, it is the redox state of metals and ligands that complex them, which then determines (i) their “unlocking” from pristine reservoirs (e.g. minerals in which they occur at trace level); (ii) their mobility on Earth through carriers such as magma, water, or vapor; and eventually (iii) their accumulation and precipitations in new phases making up ore deposits.
Redox reactions involve a coupled transfer of electrons, so for any oxidation (loss of electrons) a reciprocal reduction (gain of electrons) occurs. Moreover, redox reactions naturally occurring on Earth involve a net chemical change that can be described not only via the exchange of electrons between ions or their complexes, but also of oxygen and/or hydrogen atoms and compounds that these can form (e.g., Cicconi et al., 2020a and references therein). Here are some examples:
(1.1) 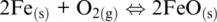
(1.2) 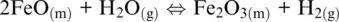
(1.3) 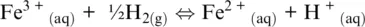
(1.4) 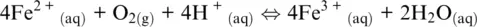
(1.5) 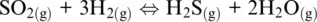
In which the subscripts s , m, aq , and g refer to solid, melt, aqueous, and gas (including supercritical fluids) phases, respectively. In the five examples above, O 2(g), H 2O (g), Fe 3+ (aq), O 2(g)and SO 2(g)are oxidizing agents, whereas Fe (s), FeO (l), H 2(g), Fe 2+ (aq)are the reducing agents.
The word oxidation was introduced by Antoine Lavoisier (1777) and indicates the chemical mechanisms in which oxygen is consumed and added to a compound. The parallel mechanism in which some other compounds loose oxygen was called reduction. The oxidation number of reaction components in Reactions 1.1to 1.5shows that some elements have a greater affinity for electrons than others and that oxygen tends to appear with oxidation number –II in its compounds (including the O 2–species in its ionic compounds). In contrast, hydrogen tends to appear with oxidation number I. Besides, hydrogen and oxygen atoms and their compounds in reaction are related to ligands making up the most important matrixes on Earth, such as aqueous solutions (including highly concentrated saline solution), supercritical fluids and gases, silicate melts or oxide, and silicate minerals making up rocks. Except for rocks, these matrixes are also important transport agents and thus redox carriers within Earth geochemical reservoirs and throughout their boundaries. Therefore, nearly all redox exchanges on Earth are related to chemical transfers involving hydrogen‐ and/or oxygen‐based half‐reactions such as (Cicconi et al., 2020a,b):
(1.6) 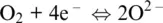
(1.7) 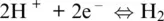
that we will call henceforth oxygen and hydrogen electrodes (e.g., Cicconi et al., 2020a; Moretti, 2020) and in which the electron exchange is made explicit. For example, Reaction 1.1can be written as the sum of Reaction 1.6and:
(1.8) 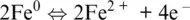
plus the formation of FeO oxides from its ions,
(1.9) 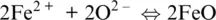
Читать дальше