Other pioneers interested in this type of surgery had also attempted the creation of artificial bladders from bowel or alternatively developing cutaneous ureterostomies [22]. Appleby (Vancouver, Canada) examined the possibility of transferring both ureters to an intact cecum draining through a sigmoid colostomy, but with limited effect [7]. Similarly, Bricker created a diversion that involved isolation of a cecal segment “to be drained intermittently of urine through a catheter” [6]. Gilchrist and colleagues reported attaining successful continence with the construction of an intra‐abdominal reservoir from isolated cecum draining via the terminal ileum [28]. However, Bricker was unable to duplicate these results and chronic leakage of urine frustrated clinicians and patients alike ( Figure 1.2) [29].

Figure 1.1 (a) Levels of transection of the ureters (U) and colon (C) and incision encompassing the vulva (V) and anus (PW) from Brunschwig’s original article. (b) Conditions at end of operation, indicating areas of peritonectomy (shaded area, P, P′, PI″, and PI‴). Midline colostomy is shown with both ureters (U and U′) implanted into the colon a short distance above colostomy. Copyright © 1948 American Cancer Society.
Source: Reproduced with permission from John Wiley & Sons Ltd. [1].
In 1944, Alfred Strauss (Chicago, USA) encouraged a young engineering student named Koenig who had an ileostomy following colectomy for ulcerative colitis to develop an ileostomy appliance. Koenig designed a slender bag with a circular faceplate to accommodate the stoma. This was held in place with a latex sealant, Koenig formed a commercial partnership with Rutzen and the device was known as the Koenig–Rutzen bag. When Bricker heard of the device, he and his colleagues began to direct their efforts toward refining the construction of the uretero‐ileal conduit [24].
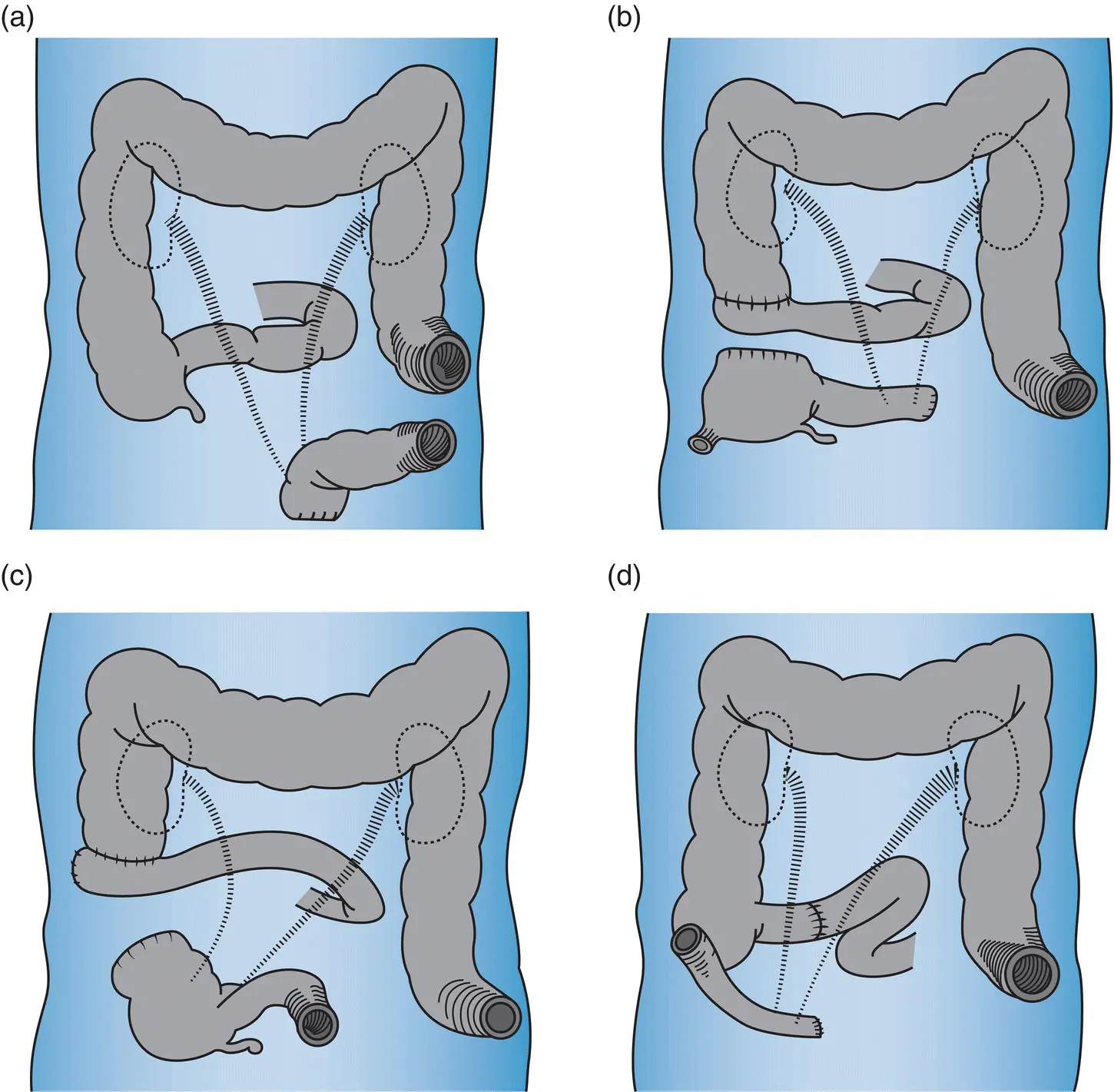
Figure 1.2 Diagram from Bricker’s original article on urinary diversion demonstrating the evolution of various intestinal reconstruction techniques, including bilateral ureteric anastomosis to an isolated segment of sigmoid colon (A), terminal ileum with cecal reservoir (B), cecum with terminal ileum for urinary drainage tract (C), and contemporary ileal conduit (D). Copyright © 1950 Surgical Clinics of North America.
Source: Reproduced with permission from Elsevier [29].
Evolution of the Uretero‐Ileal Conduit
By the late 1950s, the ileal conduit became the established urinary diversion technique, and the high mortality and morbidity rates associated with pelvic exenteration began to decline [30]. In particular the procedure avoided the complications of implanting ureters into an intact colon and could be fashioned from ileum that was undisturbed by any pre‐existing radiotherapeutic field [31]. Despite these benefits, the complex nature of exenterative surgery made significant postoperative complications associated with urinary diversion were considered unavoidable, particularly the development of urinary fistulas [15, 32]. Brunschwig observed that, in patients who survived > 5 years “the most frequent subsequent cause of death is the deterioration of the diverted urinary tract” [33]. He advocated continuous surveillance of the urinary diversion and for the early use of temporary or permanent nephrostomy tubes for any evidence of obstruction [33].
Today, en‐bloc cystectomy is required in approximately half of all patients undergoing pelvic exenteration [34–37]. Despite much progress, postoperative urological complications remain a major cause of morbidity, prolonging hospital admission and impacting on quality of life [35]. Major complication rates between 9 and 24% are reported, with urinary leak rates occurring in 7–16% of patient [35–37]. Newer techniques for continent urinary diversion, such as the internal ileal pouch reservoir [38, 39], remain controversial. Alternatives like the Indiana pouch and the Miami pouch are suitable in highly selected patients [40, 41].
Subspecialization and Partial Exenteration
The synchronous abdomino‐perineal pelvic exenteration performed by the majority of exenterative units today was adapted from the technique for LARC described by Schmitz (Chicago, USA) in 1959 [42]. Over time it was recognized that the malignancy did not always extend to all of the adjacent pelvic organs. Consequently, partial exenteration was described, preserving urinary and/or rectal function. The later part of the twentieth century also saw the intensification of surgical subspecialization, driven in part by returning surgical veterans from World War II who had gained experience in specialties such as orthopedics and plastic and reconstructive surgery. The rapid subspecialization that ensued, combined with major advances in perioperative care, including intensive care and cardiac monitoring contributed to the progress seen in exenterative surgery ( Figure 1.3) [2].
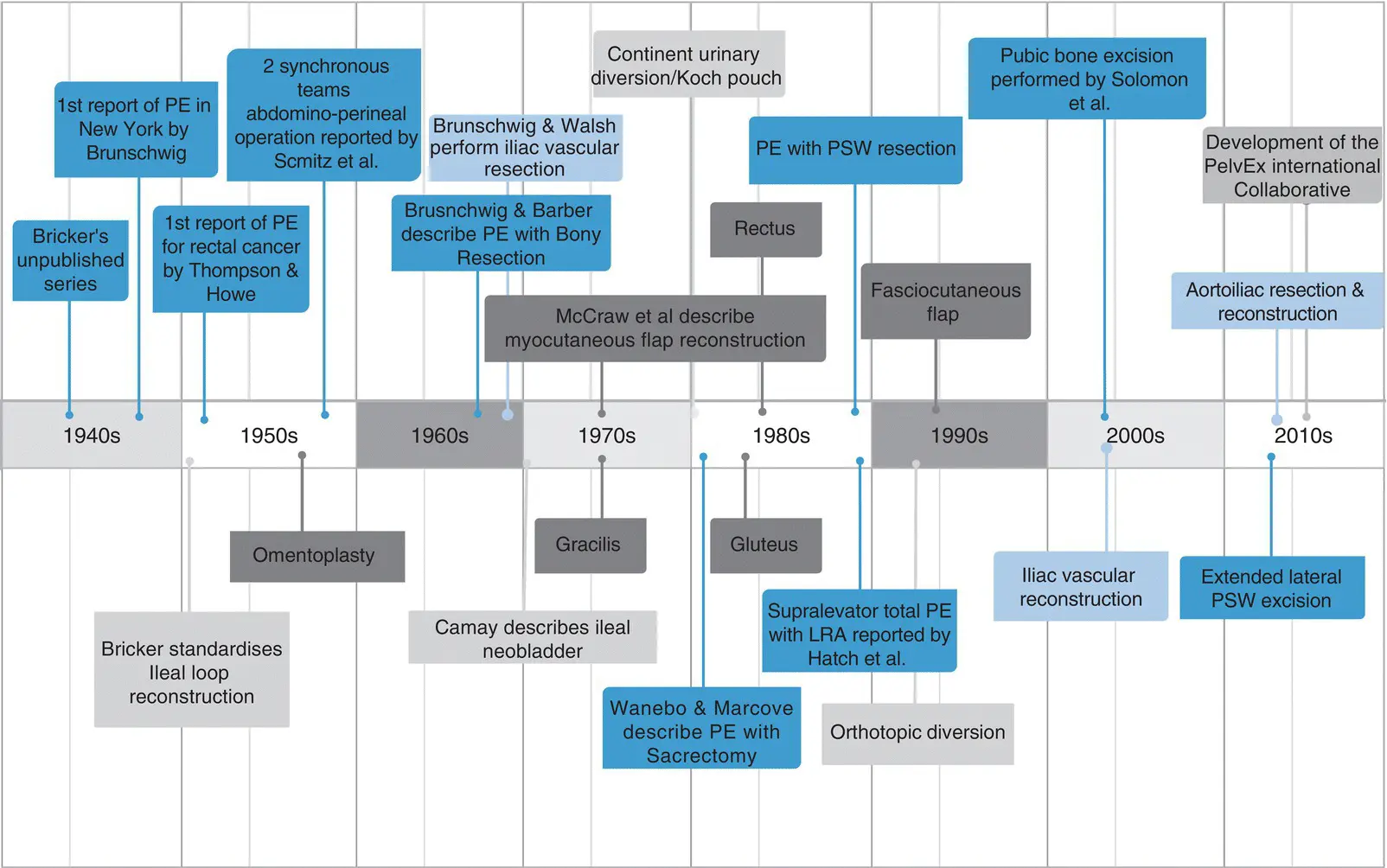
Figure 1.3 Evolution of pelvic exenterative surgery.
Composite Pelvic Exenterations
The development of compartmentalization of the pelvis and of partial exenteration resulted in more targeted approaches Bone resection was necessary for tumors involving the sacrum, coccyx, ischium, pubic symphysis, and/or ischiopubic rami [2]. Recent collaborative data show that bone resection (where needed) along with R0 margins are the most important factors influencing overall survival following PE for LRRC [5]. Disease proximal to the S1/S2 level was considered unresectable in many centers, and this represents another challenge [43–46].
Brunschwig and Barber reported a series of 28 patients, perioperative mortality was 29%, with five‐year survival of 15% [47]. These initial outcomes discouraged many from pursing en‐bloc bone resection. Research and better operative techniques developed for the management of sacral chordomas rekindled interest in composite PE in the 1980s [48]. Wanebo and Marcove (Charlottesville, USA) described the abdominal‐trans‐sacral approach for resecting LARC with sacral extension in 1981 ( Figure 1.4) [49]. The initial dissection of the intrapelvic organs was accomplished through the traditional anterior approach followed by resection of the sacrum with the patient repositioned lying prone [46, 49]. Takagi and colleagues (Nagoya, Japan) encountered no postoperative mortality with this technique [50].
These outcomes stimulated research into the role of composite sacral resection for LARC and led to various units undertaking more radical resections, reporting morbidity rates between 40 and 91%, with < 5% perioperative mortality and five‐year survival of almost 50% [51–55]. In recent years, specialist units developed techniques for en‐bloc partial sacral resection. Hemisacrectomy, a procedure involving resection of the anterior cortex of the sacrum to preserve the sacral nerve roots, and segmental sacrectomy are alternatives [55–59].
Lateral Pelvic Sidewall Resection
Brunschwig and Walsh described “resection of the great veins of the lateral pelvic wall” to gain clearance for advanced gynecological tumors in the late 1940s [60]. However, extension of pelvic cancer into the pelvic sidewall was traditionally been considered contraindication to resection. Due to the technical difficulty of safely attaining an R0 resection margin. Efforts at vascular reconstruction were hampered by the procedure being frequently preformed in a grossly contaminated and often previously heavily irradiated field [61]. Due to these poor early outcomes, few undertook such radical resections until very recently [62].
Читать дальше















