Microbial Interactions at Nanobiotechnology Interfaces
Здесь есть возможность читать онлайн «Microbial Interactions at Nanobiotechnology Interfaces» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Microbial Interactions at Nanobiotechnology Interfaces
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Microbial Interactions at Nanobiotechnology Interfaces: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Microbial Interactions at Nanobiotechnology Interfaces»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Microbial Interactions at Nanobiotechnology Interfaces — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Microbial Interactions at Nanobiotechnology Interfaces», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Metal NMs include silver NPs, copper NPs, and iron NPs. Silver NPs are one of the well‐known antimicrobial materials that have been used in various healthcare, industrial, and medicinal sectors, their mechanism of action being the toxic effect of Ag +ions exerted by interacting with sulfhydryl groups in proteins. Further, silver ions also inhibit DNA replication and also affect the cell membrane integrity, thus compromising the permeability (Feng et al., 2000). Wigginton et al. (2010) showed that the Ag NPs have high affinity for tryptophanase protein of E. coli . It is evident from the literature that the enzyme tryptophanase is crucial for the production of indole from tryptophan amino acid where indole plays a crucial role behind the multidrug exporters and also acts as a signaling molecule in biofilm formation. It was clearly evident from the study that the binding of Ag NPs deactivated the enzyme which could make the bacteria prone to effect of drugs and other antimicrobial materials (Wigginton et al., 2010). Ag NPs exhibited bactericidal activity by inactivating the enzyme phosphomannose isomerase in bacteria, which converts mannose 6 phosphate to fructose 6 phosphate. This conversion is one of the crucial steps in glycolysis of bacterial sugar metabolism (Dakal et al., 2016; Sundar & Kumar Prajapati, 2012). In another study, Elechiguerra et al. (2005) showed that the interaction of Ag NPs with HIV‐1 was not random but based on the structure of virus envelope. The envelope consists of a protruding gp120 glycoprotein connected to intracellular matrix protein p17 through a transmembrane glycoprotein gp41. Importantly, HIV‐1 binds to CD4 receptor sites on the host cell using gp120 protein knobs. It was observed in this study that Ag NPs attach to the HIV‐1 surface by sulfur‐containing residues of the gp120 protein (Elechiguerra et al., 2005). Next to silver NPs, copper NPs have gained attention in the past few years as cost‐effective alternatives to the former. Copper NPs also exert a mechanism similar to that of silver. They act by damaging the bacterial cell wall and disrupting the DNA structure (Raffi et al., 2010).
1.7.2 Metal Oxides
Similar to metal NMs, oxides of metal also exhibit antimicrobial activity through the metal ions released from the system. The mechanism of action is the ROS production induced by metal ions or by the accumulation of NPs inside the cells. Most metal oxides are semiconductors in nature with larger bandgap energy. Among the metal oxides, ZnO (3.2 eV) has the highest bandgap energy similar to TiO 2,which gets activated at a wavelength of about 390 nm to induce ROS production. CdO has a bandgap energy of about 2.1 eV with activation wavelength at 590 nm ( Table 1.2).
Among the metal oxides, the most widely used are TiO 2, ZnO, CuO, and MgO for antibacterial activity. A vastly studied metal oxide is TiO 2,which is used in various industrial and environmental applications such as in sunscreen preparation, implant coatings, and removal of water and air contaminants. The antimicrobial efficiency of a system depends on the wavelength of light used for activation, the intensity of light, concentration, interaction time, temperature, and target microbes (Markowska‐Szczupak, Ulfig, & Morawski, 2011). Efficacy of the TiO 2system depends on the photoinduced generation of ROS, which happens effectively at the oxide anatase phase. When light of energy equal to or higher than the bandgap energy (3.22 eV, anatase phase) is exposed, photocatalytic activation produces an energy‐rich electron–hole pair. The electron produced is transferred to reducible species such oxygen to generate free radicals like superoxides O 2 −. In a similar way, it induces the production of hydroxyl radicals and hydrogen peroxide in acidic conditions (Kühn et al., 2003). A schematic representation of photoactivated ROS generation and antimicrobial property of NMs is given in Figure 1.2as described by Gardini et al. (2018)
Table 1.2 Bandgap energy and activation wavelength for various metal oxide NMs (Gardini et al., 2018).
| S. No | Material | Bandgap energy (eV) | Activation wavelength (nm) |
|---|---|---|---|
| 1 | CdO | 2.1 | 590 |
| 2 | Fe 2O 3 | 2.2 | 565 |
| 3 | WO 3 | 2.8 | 443 |
| 4 | TiO 2 | 3.2 | 387 |
| 5 | ZnO | 3.2 | 390 |
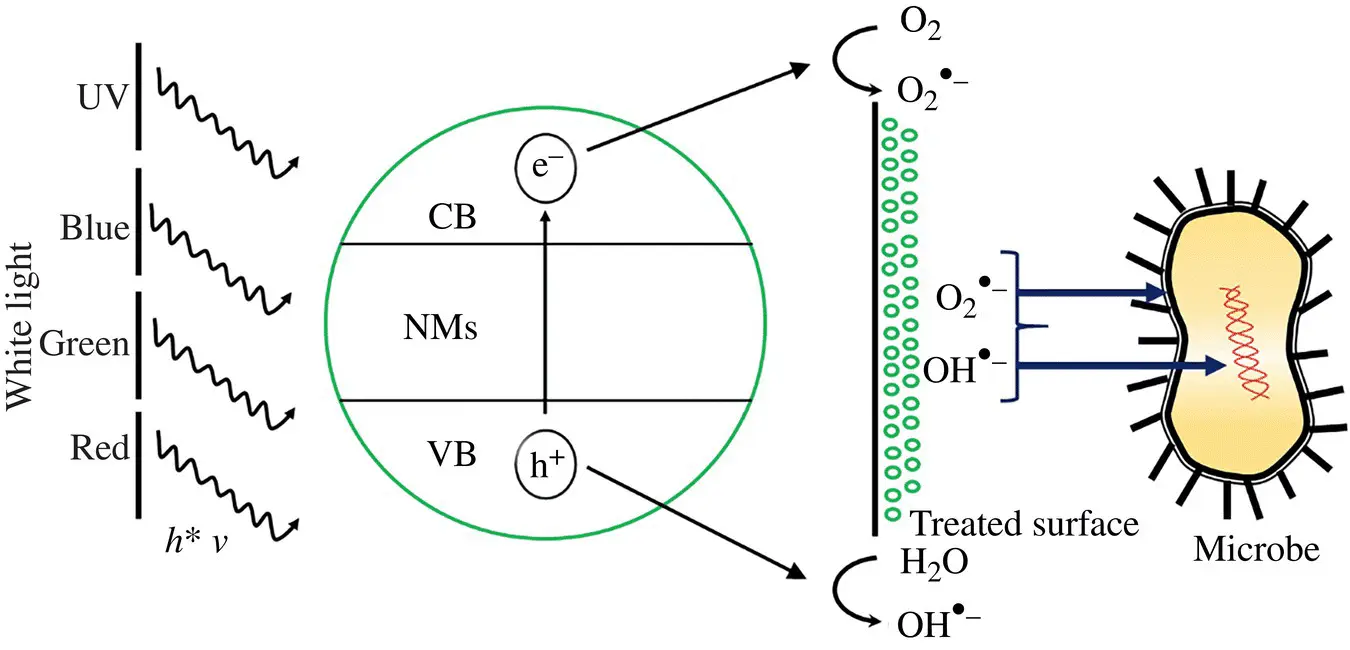
Figure 1.2 Schematic representation of photoactivated ROS generation and antimicrobial property of NMs.
In a similar way, ZnO, a semiconductor with larger bandgap energy, is applied in coatings, paints, and sunscreens. Upon exposure of UV light, photocatalysis induces the ROS production, which is responsible for its antimicrobial effect. Here, the roughness of the system depends on the surface defects. Efficacy of the ZnO NP system increases with decrease in size where the roughness of the particle along with its high surface area causes the disruption of the microbial cell wall (Padmavathy & Vijayaraghavan, 2008). ZnO NMs have also been reported to interact with some disease target proteins. Chatterjee et al. (2010) studied the effect of ZnO NPs over periplasmic domain structure of ToxR protein of Vibrio cholerae . ToxR protein plays a critical role in the regulation of expression of many virulence factors of the bacteria. It was observed that the binding of the protein ToxR to ZnO NPs' surface reduced the stability of protein where it was more susceptible to denaturation. Further, significant change in the structure of the protein was also observed (Chatterjee et al., 2010).
1.7.3 Carbonaceous NMs
At the nanoscale, carbon forms different allotropes, which include graphene, carbon nanotubes, fullerenes, and nanodiamonds where each of them exerts inherent unique properties. The antimicrobial effect of carbon NMs such as graphene, graphene oxide, reduced graphene oxide, and carbon nanotubes depends on the level of cell wall disruption and amount of ROS induced in the microbial cells (Liu et al., 2011). Carbon materials hold the advantages of commercial viability and environmental safety in comparison to conventional NPs such as silver and other metal NPs.
1.7.4 Cationic Polymer NMs
Polymers with an inherent positive surface charge or added with positively charged moieties are called cationic polymer NMs. The most commonly used cationic polymer in antimicrobial application is chitosan (Rekha Deka, Kumar Sharma, & Kumar, 2015). Chitosan is a cationic polysaccharide derived from chitin by partial deacetylation. The positively charged surface moieties aid in the interaction of NM with negatively charged bacterial cell wall, which causes the rupture of membrane and subsequent cytoplasmic content leakage (Qi et al., 2004). The efficacy of the system relies on the pH, degree of deacetylation, molecular weight, and presence of other substances such as proteins, lipids, and metal ions. The major disadvantage of chitosan‐based nanosystems is their poor solubility at neutral pH causing them to precipitate in culture medium.
In general, the efficacy of the antimicrobial NMs depends on the interaction of the material with bacteria and the mechanism of action. Further, the interaction of NMs with bacteria depends on a few crucial factors such as electrostatic attraction, van der Waals forces, receptor–ligand interaction, and hydrophobic interaction. Understanding the basics of the NMs' interaction with the microbial cell is likely to pave way for the design of novel antimicrobial agents with crucial insight into their toxicity and mechanism of action. In the following sections, we discuss the interaction of NMs with microbial cells and the mode of action.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Microbial Interactions at Nanobiotechnology Interfaces»
Представляем Вашему вниманию похожие книги на «Microbial Interactions at Nanobiotechnology Interfaces» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Microbial Interactions at Nanobiotechnology Interfaces» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












