Microbial Interactions at Nanobiotechnology Interfaces
Здесь есть возможность читать онлайн «Microbial Interactions at Nanobiotechnology Interfaces» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Microbial Interactions at Nanobiotechnology Interfaces
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Microbial Interactions at Nanobiotechnology Interfaces: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Microbial Interactions at Nanobiotechnology Interfaces»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Microbial Interactions at Nanobiotechnology Interfaces — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Microbial Interactions at Nanobiotechnology Interfaces», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
1.2 Synthesis of Nanomaterials
NMs can be synthesized by a number of methods that are grouped into two different categories: (i) bottom‐up and (ii) top‐down method. Schematics of typical methods for synthesis of NMs are given in Figure 1.1as described by Ealias and Saravanakumar (2017).
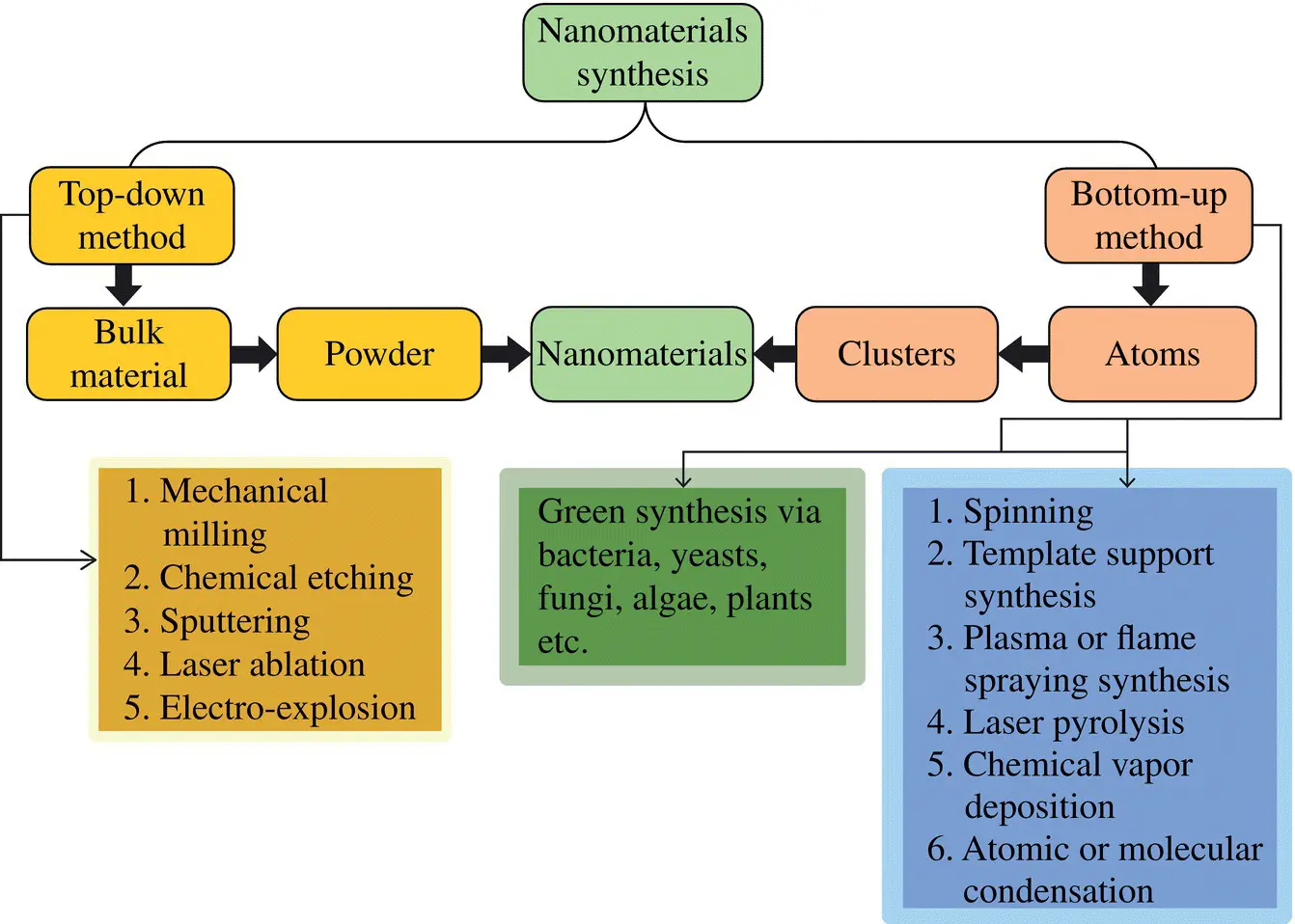
Figure 1.1 Schematics of typical methods for synthesis of NMs.
1 Bottom‐up method: It is a constructive method wherein the atoms build up clusters that in turn form the NMs. This category includes methods such as sol–gel, spinning, chemical vapor deposition, pyrolysis, and biosynthesis.
2 Top‐down method: On the contrary, the top‐down method is a destructive method where the bulk materials are reduced into nanoscale materials. It includes methods such as mechanical milling, nanolithography, laser ablation, sputtering, and thermal decomposition. The typical method of synthesis for various NMs is given in Table 1.1.
1.3 Classification of NMs
In nature, NMs are built by nanoscale or submicron‐sized blocks that exhibit size‐dependent effects. Over the last two decades, a number of NMs or nanostructured materials have been developed and a lot of new developments are underway. The abundant increase in the number of NMs has set forth the need for the classification of these materials. An understanding of the classification would give insight into the interaction of NMs with various surfaces and resultant functionality of the NMs. The first classification of NMs was given by Gleiter (2000) in which the materials were classified on the basis of crystalline forms and chemical compositions. The scheme subdivided the materials into three classes where each class has four kinds of materials (Gleiter, 2000). However, the list of Gleiter was not considered complete as it failed to take account of 0D and 1D NMs such as fullerenes and nanotubes into the classification.
Table 1.1 Various approaches for NM synthesis (Ealias & Saravanakumar, 2017).
| S. No | Category | Method | NMs |
|---|---|---|---|
| 1 | Bottom‐up | Sol–gel | Metal and metal oxide and carbon NMs |
| Spinning | Organic polymers | ||
| Chemical vapor deposition | Carbon and metal NMs | ||
| Pyrolysis | Metal oxide and carbon NMs | ||
| Biosynthesis | Metal and organic polymer NMs | ||
| 2 | Top‐down | Mechanical milling | Metal, metal oxide, and polymeric NMs |
| Nanolithography | Metal NMs | ||
| Laser ablation | Carbon and metal oxide NMs | ||
| Sputtering | Metal NMs | ||
| Thermal decomposition | Metal oxide and carbon NMs |
1.3.1 Classification Based on Dimensions
Later, Pokropivny and Skorokhod (2007) proposed a new scheme of NM classification where the dimensionality (shape and size or form) of the NMs was considered as a primary criterion. In general, nanostructures are structures with at least one dimension d equal to or less than 100 nm, which is considered as d *. The value d * is always dictated by physical phenomena such as path length of phonons and electrons, diffusional length, length of de Broglie wave, penetration length, and correction length. According to the scheme, NMs were classified into four major categories: 0D, 1D, 2D, and 3D (Pokropivny & Skorokhod, 2007).
1.3.1.1 Zero‐Dimensional NMs
Zero‐dimensional NMs are defined as materials where all the three dimensions are confined to the nanoscale (1–100 nm). The same definition could also be stated on basis of the movement of electrons along the dimensions of the NMs. Zero‐dimensional materials are materials where the electrons are merely entrapped in a dimensionless space without any possible movement (Jeevanandam et al., 2018). The best examples for 0D NMs are NPs and quantum dots. Over the past decades, 0D materials have gained a lot of interest where a number of methods have been designed to fabricate 0D NMs with precise dimensions. 0D NMs can be crystalline or amorphous in nature. They may be mono‐ or polycrystalline, single‐ or multi‐element, and may exist in various forms (shapes and sizes). These materials have found application in a number of fields such as solar cells (Lee et al., 2009), light‐emitting diodes (Stouwdam & Janssen, 2008), single‐electron transistors (Mokerov et al., 2001), lasers, therapeutics and diagnosis (Azzazy, Mansour, & Kazmierczak, 2007).
1.3.1.2 One‐Dimensional NMs
One‐dimensional NM are the materials where one of the dimensions is in macroscale with other two dimensions confined to the nanoscale (<100 nm) (Xia et al., 2003). Herein, the electrons can move across one axis freely whereas they entrapped in other two dimensions of the NMs (Jeevanandam et al., 2018). These 1D NMs are ideal choice for studying the dimension‐dependent activity of the materials. Similar to 0D NMs they also can be amorphous or crystalline, mono‐ or polycrystalline, ceramic, polymeric or composite materials of different shapes and sizes. 1D materials such as nanotubes, nanowires, and nanofibers have attracted a lot of interest in the development of hierarchal nanostructures such as nanofilms, nanosheets, and nanoribbons with profound applications in the field of optoelectronics and nanoelectronics (Cui et al., 2001; Kong et al., 2000).
1.3.1.3 Two‐Dimensional NMs
Materials with one of the dimensions in the nanoscale (≤100 nm) and the other two dimensions in macroscale are called 2D materials. Here the electrons are confined in one direction whereas they can move across in other two axes freely (Jeevanandam et al., 2018). Similar to 0D and 1D, 2D materials can also be amorphous or crystalline, poly or monocrystalline, single‐ or multi‐element, which also exist in different forms. 2D materials such as nanosheets, nanofilms, and nanoribbons have shown promising applications in the fields of optoelectronics, sensors, and biomedicine (Weaver et al., 2014).
1.3.1.4 Three‐Dimensional NMs
Herein, the materials have all the three dimensions in macroscale but are comprised of uniformly distributed nanometer‐sized grains. Hence, the movement of the electrons can be free across all the three dimensions without any confinement (Jeevanandam et al., 2018). 3D NMs also called bulk NMs are widely used in catalysis, electrodes, and magnetic materials. Nano balls, nano coils, and nanoflowers are typical 3D NMs that have high surface area and can provide maximum adsorption sites for all the molecules in a small‐area framework (Shen et al., 2008).
1.3.2 Classification Based on Chemical Compositions
Similar to dimension, the composition of NMs also plays a vital role in deciding their activities and application. On the basis of composition, NMs are classified into four subcategories, namely: (i) carbon‐based NMs, (ii) organic NMs, (iii) inorganic NMs, and (iv) composite NMs.
1.3.2.1 Carbon‐Based NMs
The NMs with carbon atoms as their backbone are called carbon‐based NMs. They can exist in different forms such as 0D (fullerenes), 1D (carbon nanotubes), 2D (graphene sheets), and 3D (diamond crystal and graphite). General methods to prepare these NM include chemical vapor deposition, arc discharge, and laser ablation. Carbon‐based NMs exist in different forms with multiple shapes such as hollow spheres, nanotubes, and ellipsoids (Jeevanandam et al., 2018). Fullerenes are carbon materials with spherical morphology where the carbon atoms are held by sp 2hybridization. A unique advantage of the fullerenes is their high symmetric property (Astefanei, Núñez, & Galceran, 2015). In general, fullerenes contains 28–980 carbon atoms where the diameter of single layer is up to 8.2 nm and for multilayered fullerenes it is about 4–36 nm (Ealias & Saravanakumar, 2017). Carbon nanotubes are 1D carbon NMs where carbon atoms are wound up to form hollow cylinders, which can also be described as an extension of fullerenes or buckyball. Carbon nanotubes can be single‐walled, double‐ or multi‐walled with thickness varying from 0.7 nm for single‐walled to 100 nm for multi‐walled CNTs. The length of CNTs generally varies from few micrometers to several millimeters (Ealias & Saravanakumar, 2017). CNTs have been exploited in various fields owing to their versatile properties such as elasticity, strength, rigidity, field emission, and electrical conductivity (Saeed & Khan, 2014, 2016). Graphene is one of the 2D carbon‐based materials formed by sp 2hybridized carbon atoms. It is a hexagonal network of carbon atoms with honeycomb atomic structure that is confined to a two‐dimensional planar surface. Graphene elucidates commendable physical, chemical, optical, and mechanical properties owing to their unique honeycomb atomic structure. These unique properties altogether make them remarkable materials that are extensively applied in the fields of electronics, optics, storage, thermal applications, photovoltaics, and composite materials (Goenka, Sant, & Sant, 2014; Pumera, 2010).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Microbial Interactions at Nanobiotechnology Interfaces»
Представляем Вашему вниманию похожие книги на «Microbial Interactions at Nanobiotechnology Interfaces» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Microbial Interactions at Nanobiotechnology Interfaces» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












