Pathology of Genetically Engineered and Other Mutant Mice
Здесь есть возможность читать онлайн «Pathology of Genetically Engineered and Other Mutant Mice» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Pathology of Genetically Engineered and Other Mutant Mice
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Pathology of Genetically Engineered and Other Mutant Mice: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Pathology of Genetically Engineered and Other Mutant Mice»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
An updated and comprehensive reference to pathology in every organ system in genetically modified mice Pathology of Genetically Engineered and Other Mutant Mice
Pathology of Genetically Engineered and Other Mutant Mice
Pathology of Genetically Engineered and Other Mutant Mice — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Pathology of Genetically Engineered and Other Mutant Mice», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Developmental Events in Embryos and Neonates
In mice, conception for all members of a litter occurs on average at GD0.5. At about GD1.0, the free‐floating zygotes (i.e. one‐celled embryos) begin the first of multiple rounds of cell division as they travel down the oviduct. Embryos enter the uterus at about GD2.5 as morulae (solid multi‐celled masses) and evolve into blastocysts with an off‐center, fluid‐filled cavity at about GD3.0. Dilation of this cavity is accompanied by differentiation of the first embryonic tissues: the inner cell mass (ICM), a crescentic group of pluripotent stem cells at one pole that will become the embryo proper, and the trophectoderm, which forms the outer wall of the blastocyst and will differentiate into the extra‐embryonic membranes (i.e. placenta). Evolution from morula to blastocyst is accompanied by increased activity of the embryonic genome and substantial strain‐specific metabolic changes.
Embryos implant in the uterine wall at about GD4.5. Prior to implantation, cross‐talk between the uterine wall and blastocyst results in production of a highly permeable, well‐vascularized endometrial layer, termed “decidua,” that promotes embryo attachment and survival [35, 36]. The decidual reaction provides the primary means for sustaining the embryos until they form their own placenta. Upon implantation, the trophectoderm proliferates, invades the decidua, and then differentiates into syncytiotrophoblasts (which border the maternal tissue) and cytotrophoblasts (which envelop the ICM). Reciprocal interactions between the embryos and decidua are essential in maintaining pregnancy [37, 38].
By GD5.0, the implanted round blastocyst morphs into an elongated “egg cylinder,” and distinct embryonic and placental features begin to form. At this stage, the ectoplacental cone appears as a triangle of extra‐embryonic tissue that invades the mesometrial decidua at one pole of the egg cylinder. Simultaneously, the ICM differentiates into the epiblast, an outer layer of columnar cells that will give rise to three embryonic germ layers (ectoderm, mesoderm, and endoderm) as well as some extra‐embryonic ectoderm and mesoderm, and the hypoblast, a thin inner layer of cuboidal cells that will produce the yolk sac (YS) endoderm and some extra‐embryonic mesoderm. Generation of germ layers by the epiblast begins on GD6.0 with the ectoderm and endoderm, with formation of the mesoderm (a process called “gastrulation”) beginning at about GD6.5 and lasting until about GD7.5.
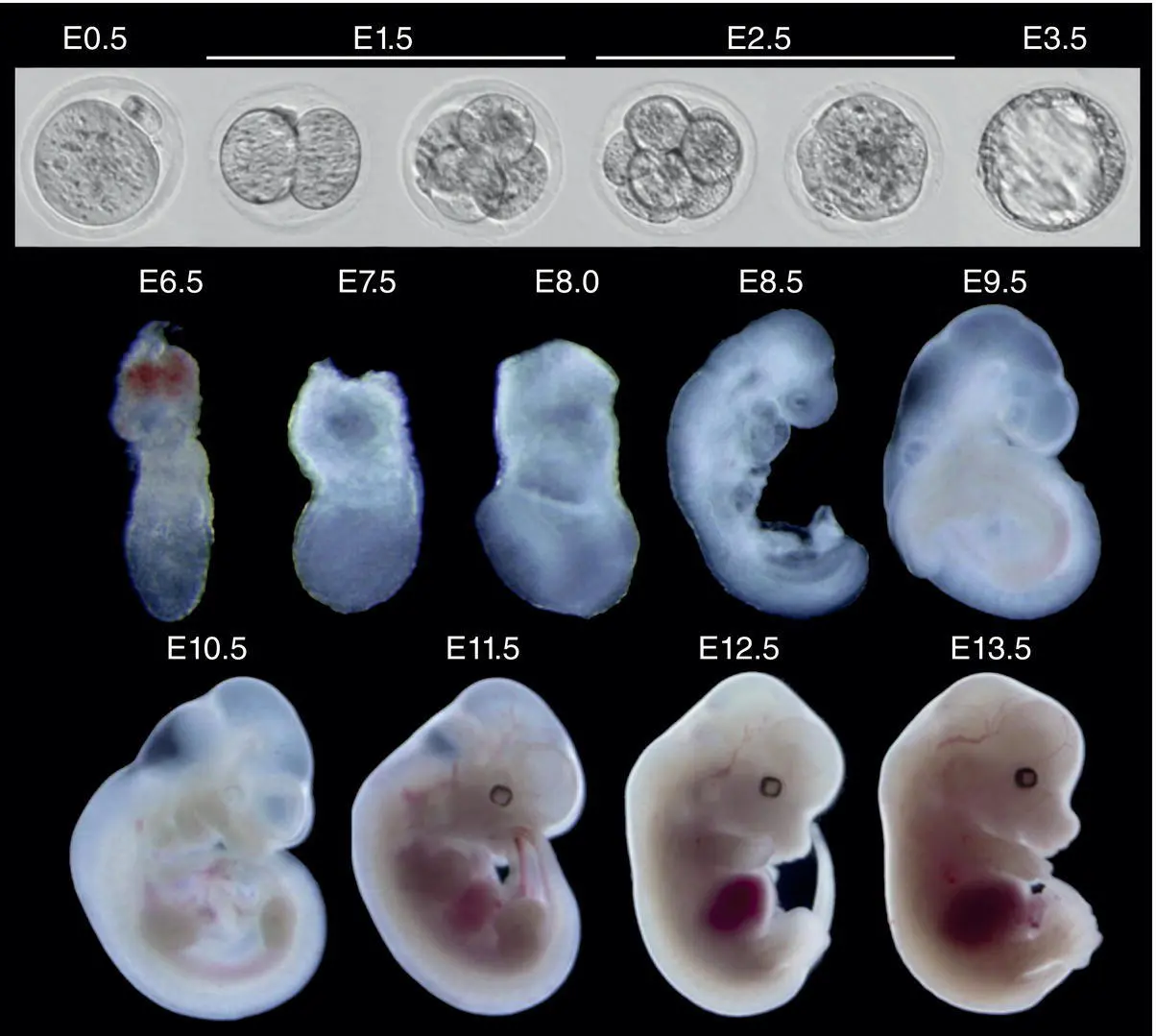
Figure 5.1 Composite image of embryonic mouse developmental stages from just after conception (at embryonic day [E] 0.5, in upper left) throughout preimplantation (E1.5 to E3.5, remainder of upper row) and during early postimplantation (E6.5) to mid‐gestation (E13.5).
Source: Dr. Yi Zhang, Harvard Medical School and the Howard Hughes Medical Institute.
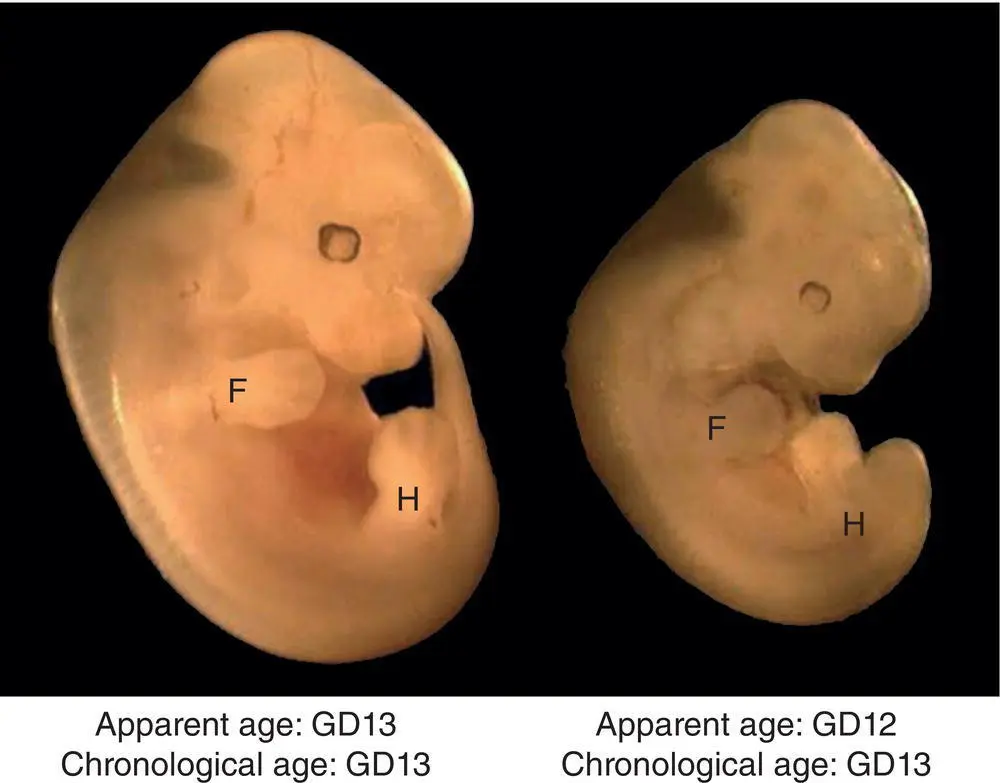
Figure 5.2 Mouse embryo littermates with a shared chronological age at gestational day (GD) 13 but having anatomic features demonstrating different developmental stages: GD13 (Theiler stage [TS] 21) on the left as shown by discrete linear gray rays denoting the sites of future digit separation on the paddle‐shaped forepaw (F) and hind paw (H) limb buds, but GD12 (TS20) on the right as shown by the absence of digital rays on the forelimb buds. Digital rays usually form on the forelimb bud at about GD12.3 and on the hind limb bud at about GD12.8.
Source: Bolon and La Perle [89] with permission of CRC Press.
Organogenesis begins at about GD7.5 and lasts until GD15.0. During this portion of embryonic development, all major organ precursors (termed “anlagen”) undergo initial specification and generation. Organ structures and the external appearance of the embryo only approximate the adult conformation at the end of organogenesis. Different organs, and parts of organs, are initiated at different “critical periods” throughout this time range so that the developmental path for most organs extends across several days [39]. These critical periods represent times of intense cell proliferation, migration, and differentiation, and thus define organ‐, region‐, and cell type‐specific times of heightened vulnerability to endogenous and exogenous insults [40, 41]. An oft forgotten fact is that for some organs, such as brain and lymph nodes, critical periods occur not only during gestation but also after birth [42, 43].
The last few days of gestation (GD15.0 to term) and the first few days after birth (up to PND7 or so) correspond to the fetal period in primate embryos. This phase represents a period of organ growth. With some exceptions, organs acquire their adult appearance at this time, although function often is decreased in neonatal and juvenile animals relative to adult systems.
Developmental Events in Placentation
The placenta is the first organ created by a newly implanted embryo (GD5.0), although its form varies considerably until the definitive placenta (DP) becomes active (GD12.5). The placenta acts as an interface for exchanging gases and organic molecules between the embryonic and maternal circulations, a hormone source that regulates maternal processes need to sustain pregnancy, and a protective environment for embryonic survival and growth. The decidua also serves as a barrier to reduce immunogenicity of embryonic proteins. The structure, function, and molecular signature of the placenta is revised greatly over time in response to the growing embryo's evolving metabolic demands [4, 16, 17, 44, 45].
The YS is the first functional placenta ( Figure 5.3). It forms shortly after implantation and acts until about GD10.5 as the sole means of gas exchange (by diffusion) and macromolecular transfer (by trophoblast phagocytosis, a process termed “histiotrophic nutrition”) [45, 46]. The YS also serves as the main hematopoietic organ in embryos from about GD8 to GD11 by virtue of the “blood islands” that form at multiple sites near YS blood vessels [8].
At about GD12.5, the DP becomes the main functionally mature placenta ( Figure 5.4). This transition is required to provide enhanced blood flow for increased nutrient exchange (a process termed “hemotrophic nutrition”). The mouse DP is a discoid, trihemochorial, chorioallantoic‐derived interface. A discoid arrangement indicates that the region for gas and nutrient exchange is a discrete mass rather than spread over the entire placental surface, while the trihemochorial organization means that maternal blood runs in blood‐filled labyrinth trophoblast‐lined channels lacking an endothelial lining (termed “sinusoids”) and embryonic blood flows through a dense labyrinth comprised of embryonic endothelial‐lined embryonic capillaries that are surrounded by one to three embryonic trophoblast layers. The chorioallantoic derivation means that the DP originates from both chorionic plate (extra‐embryonic ectoderm) and allantois (extra‐embryonic mesoderm) [45].
The late‐gestation placenta consists of multiple distinct regions, which are discerned based on their location and distinct cell composition ( Figures 5.3– 5.5). The amnion is a thin, translucent membrane that envelops the embryo. The next membrane further from the embryo is the YS, which now is highly vascular and covered on its outer surface by an acellular matrix termed Reichert's membrane (RM). The allantois is a thin, irregular column extending from the ventral abdomen to the flat bottom surface of the DP disc; this allantoic bridge eventually forms the umbilical cord. The DP disc is partitioned into distinct areas. The chorion is the red central plaque that serves as the site where the allantois fuses. The labyrinth is a dense spongy mesh covering the chorion. The labyrinth consists of embryonic capillaries and maternal sinusoids arranged in tortuous, parallel channels to maximize countercurrent blood flow for the most efficient nutrient transfer [47]. The labyrinth represents about 50% of the DP mass once the organ reaches its maximal size at about GD12.5. The junctional zone (JZ) is the middle layer located between the labyrinth and uterine wall. Blood vessels in the JZ contain only maternal blood. A trophoblast giant cell layer sits atop the JZ as a discontinuous, thin border that abuts the decidua basalis. As gestation progresses, the outer layers contain growing numbers of periodic acid Schiff (PAS)‐positive glycogen cells [48]. Specific trophoblast populations in the DP exhibit distinct patterns of molecular expression, which can shift over the course of gestation.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Pathology of Genetically Engineered and Other Mutant Mice»
Представляем Вашему вниманию похожие книги на «Pathology of Genetically Engineered and Other Mutant Mice» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Pathology of Genetically Engineered and Other Mutant Mice» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












