Mouse models also support the role of BMP-2 during bone regeneration. 125Molecular screening approaches have revealed a long list of growth and differentiation factors that are differentially expressed during bone regeneration, in particular fracture healing, that play a major role in bone formation. 54For example, BMP-4 126and BMP-7 127have no effect on fracture healing, but Wnt signaling is crucial for bone regeneration, based on observing with a sclerostin antibody and sclerostin knockout models. 4The Hedgehog signaling pathway also plays a critical role in osteoblasts during fracture repair. 6While it is obviously the orchestrated interplay of a large spectrum of local and systemic signals that drives osteoblastogenesis, and thus bone regeneration, there are growth factors such as BMP-2 that are not only supportive but also essential for proper bone regeneration, and thus likely also for graft consolidation. However, considering the complex interplay of immune cells, endothelial cells, osteocytes, and osteoclasts in controlling bone formation, many molecular mechanisms remain to be discovered.
Histology has provided insights into the defect sites, showing that osteoclasts are already active a few days after the injury, and that bone formation by osteoblasts is clearly visible 10 days after implant insertion in a pig model. 131The new bone grows fairly rapidly, at approximately 10 µm per day, and sprouts into the defect area. Then, lamellar bone is formed on the surface of the woven bone, which overall is independent of osteoclasts and is thus strictly in an anabolic phase until bone remodeling is initiated. Finally, the woven bone and the primary lamellar bone are replaced by secondary lamellar bone, which is the final stage of bone regeneration, and bone remolding takes over. What histology convincingly demonstrates is that the new bone grows into an area rich in blood vessels, but without touching them. 131Considering the three choices of osteoblasts – to become an osteocyte, to become a lining cell, or to die – a supply of new osteoblasts to drive bone regeneration seems mandatory. The close proximity of osteoblasts has always pointed toward blood vessels as the source of the mesenchymal progenitor cells, but evidence was scarce. Today, advanced mouse models have supported this hypothesis, e.g. by showing that only a certain type of endothelial cells (H-type) is associated with osteogenic precursors, which resemble pericytes but are perhaps a distinct population. 78,134Blood vessels in the growing long bones are rich in osteogenic precursors, and are predetermined as they express the differentiating marker osterix. 78Blood vessels in the bone marrow, however, do not carry this cell population. It is reasonable to suggest, under the premise that (to some extent) bone regeneration recaptures bone development, that these osteogenic blood vessels also sprout into the defects after implant insertion or bone augmentation. Moreover, Prx1-Cre mouse models support the role of the periosteum as a rich source of osteogenic cells. These cells can efficiently contribute to cartilage and bone formation upon injury. 41This knowledge now has to be translated into higher animals and its clinical relevance determined.
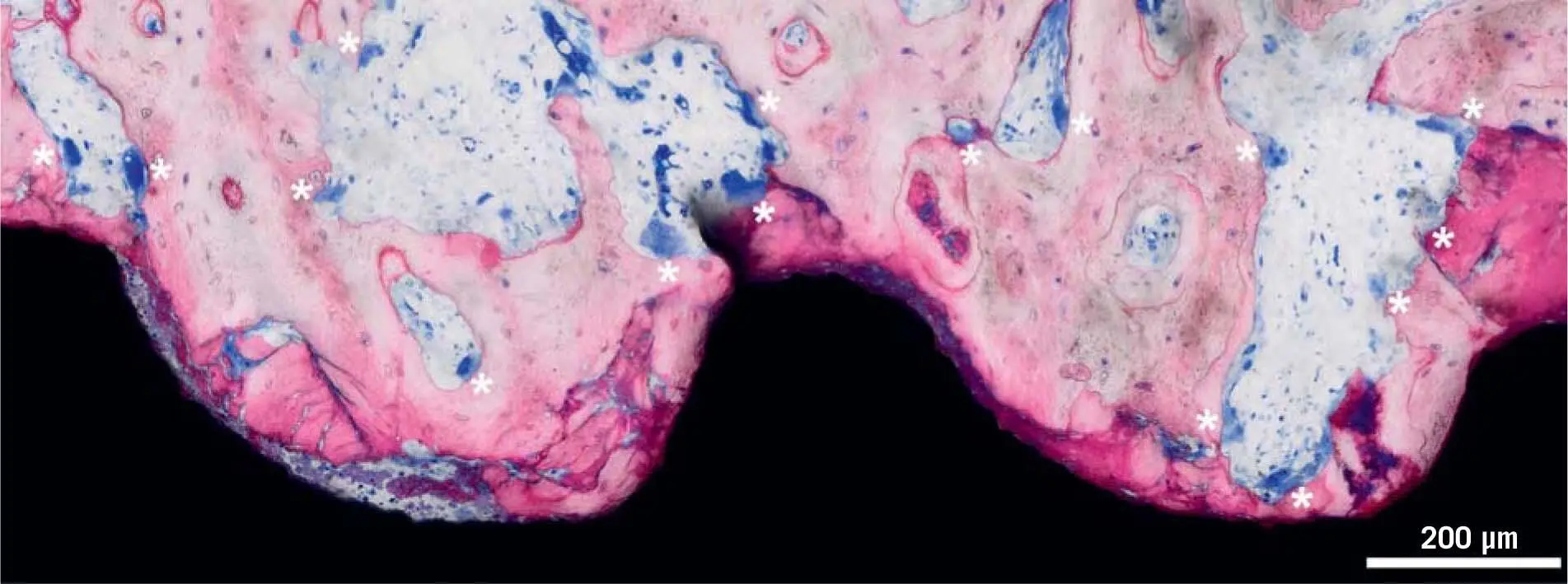
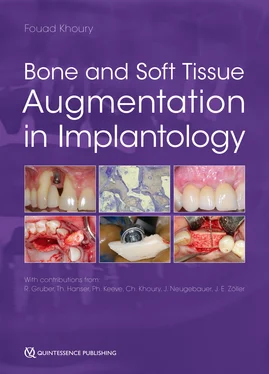
Fig 1-6Dental implant after 5 days of osseointegration in a pig jaw, from a study by Vasak et al. 131Five days after implant placement, close to the implant surface, the bone is fragmented, squeezed, and heat damaged (dark pink). Osteocytes in the vicinity are dead and dying. Osteoclasts (white asterisks) are digging bone channels, sprouting from existing bone canals (osteons) to reach and resorb the damaged bone. They are about to reach the most damaged bone close to the implant and will soon remove it.
If the overall hypothesis is correct, the formation of this subtype of endothelial cells carrying the osteogenic cells is essential for bone regeneration, and thus also for regenerative dentistry. However, since the pioneer findings with parabiosis experiments, 22there is good evidence that blood vessels provide the progenitors of osteoclasts. Here, the bone marrow is irradiated and thus osteoclastogenesis is impaired; however, it is regained when the circulation is connected to a vital mouse, so that osteoclast progenitors have to be carried via the bloodstream. Taken together, the blood vessels are key for osteoblastogenesis and osteoclastogenesis – and consequently also for bone regeneration.
1.3.1 Osseointegration of dental implants
It is known from preclinical histologic investigations in minipig 131and mandibular canines, 9but also by measuring implant stability in a clinical setting, 108,132that within the first week, resorption of the peri-implant bone dominates the scene, before bone formation takes over ( Fig 1-6). This early catabolic process is required for the removal of micro-damaged necrotic bone, which is characterized by dying osteocytes. After around 1 week, the osteoclasts have disappeared, leaving behind an osteophilic surface onto which new bone is deposited ( Fig 1-7). 9,131Small defects, as they occur between the local bone and the threads of the implants, are bridged with new bone. These relatively small distances are known as jumping distances. 11Primary woven bone formation shows a typical picture, with blood vessels in the center of an open ring of new bone ( Fig 1-8). This image supports the assumption that the origin of the cells required for bone formation is based on pericytic progenitor cells of sprouting blood vessels. 78,134This bone is immature (woven bone). 9Subsequently, lamellar bone will strengthen the woven bone that later undergoes modeling and remodeling.
Fig 1-7Dental implant after 10 days of osseointegration in a pig jaw, from a study by Vasak et al. 131Ten days after implant placement, feeble trabeculae of new bone have already replaced the damaged old bone. New bone continues to grow. Batches of osteoclasts are resorbing the remaining damaged bone.
Modeling refers to the functional adjustments based on the reaction of the bone to biomechanical stimuli according to Wolff’s law and Frost’s Mechanostat theory. 44We are beginning to understand today how resident bone cells perceive and translate mechanical energy into biologic signals. These signals transiently uncouple the remodeling equilibrium of osteoblasts and osteoclasts; otherwise, no structural change of bone anatomy is possible. 96Remodeling, then, ensures the preservation of bone quality and long-term implant success. According to current hypotheses, the necrotic bone areas created during loading are resorbed by osteoclasts and are immediately replaced by osteoblasts, which was originally postulated by Frost 44and has now been proven to involve the apoptotic and necrotic death of osteocytes. 65,66Osseointegration is therefore not only the transition from mechanical primary instability to biologic secondary stability due to bone regeneration; 86it also requires the continual maintenance of bone quality through remodeling. Bone regeneration and bone remodeling are not necessarily subjected to the same regulatory mechanisms; for example, bone formation during early fracture healing can take place without the resorptive activity of osteoclasts, 50,85while bone remodeling is strictly based on the coupled effect of osteoclasts and osteoblasts. 112What is true for osseointegration is also observed during graft consolidation – the osseointegration of autografts.
Читать дальше