1 ...6 7 8 10 11 12 ...33 In the second category, surfactants are normally grouped according to the presence or absence of formal electrostatic charges in the hydrophilic moiety of the molecule. Thus, cationic surfactants contain formal positive charges (+) on the polar head of the surfactant molecule and are systematically escorted by negative counterions that neutralize the charges. Anionic surfactants have negative formal charges (−), an example of this latter is represented in Figure 2.1. The third class of ionic surfactants is those species comprising both positive and negative charges in the same body (±). These species are known as inner salts or zwitterions (from German zwitter = hybrid). The last case of this classification is the absence of formal charges in the surfactant molecule, so these are called nonionic surfactants.
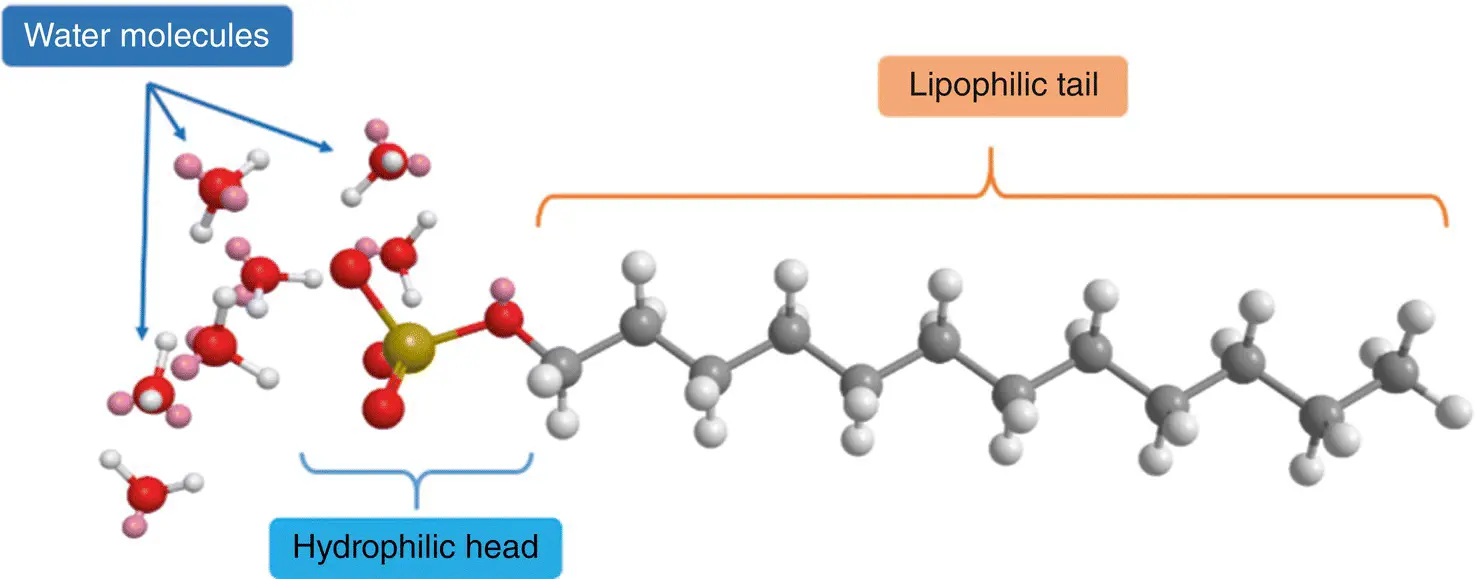
Figure 2.1 3D chemical structure of lauryl sulfate as an example of a surfactant molecule. The hydrophilic head of sulfate (SO 4 2−) is surrounded by water molecules, while the lipophilic tail avoids any contact with water.
Source: Based on [1].
Table 2.1 Classification of surfactants by origin, ionic status, and hydrophilic–lipophilic balance.
| Category |
Example |
| I. Origin |
Synthetic |
Nonylphenol ethoxylates |
| Oleochemical |
Lauryl alcohol ethoxylates |
| Biosurfactants |
Sophorolipids |
| II. Electrostatic status |
Oonic |
Cationic |
Ammonium salts |
| Anionic |
Lauryl sulfates |
| Zwitterionic |
Betaines |
| Nonionic |
Oxirane and 2‐methyoxirane copolymers |
| III. Hydrophilic–lipophilic balance |
01–03 |
Antifoaming agents |
| 03–08 |
w/o emulsifiers |
| 07–10 |
Wetting agents |
| 08–16 |
o/w emulsifiers |
| 13–16 |
Detergents |
| 16–19 |
Solubilizing agent |
Finally, surfactants can be ranked according to their amphiphilic nature, otherwise by their hydrophilic–lipophilic balance (HLB). Although there are a significant number of theoretical and experimental approaches, the most used HLB system is the Griffin’s [3]. In a simplistic way, HLB values are estimated from the division of the mass of the hydrophilic fragment by the mass of the entire molecule, and the resulting quotient is then multiplied by a conventional value of 20. HLB can predict whether a surfactant will behave as an emulsifier, solubilizer, dispersant, or other; therefore, this system has been a useful guide to formulate products containing conventional surfactants for specific applications, and certainly, will be just as convenient for the case of BS. The hydrophilic part of BS is normally constituted by carbohydrates, amino acids, proteins, phosphates, carboxylic acids, or alcohol motifs; and these can be ionic or nonionic. The lipophilic part commonly is long chains of carbon atoms, just as in fatty acids. Both molecular components, hydrophilic and lipophilic, are assembled via linking biochemical functionalities, e.g. ethers (C−O−C), amides (N−C=O), and esters (O−C=O). According to the nature of each moiety hydrophilic and lipophilic, BS are commonly classified in the following groups: (i) glycolipids, (ii) lipopolysaccharides, (iii) lipopeptides, (iv) phospholipids, and (v) fatty acids; each one with specific physicochemical characteristics and physiological roles [4, 5]; for example, Emulsan and other complex lipopolysaccharides are polymeric BS with known emulsification capabilities.
From all the types of BS, glycolipids have the greatest opportunity to be manufactured on a large scale due to the high yield of obtention compared to other BS such as lipoproteins. It is clear that BS produced in higher yields will represent a lower cost for production [6]. This is why glycolipids have captured our attention for this chapter. Glycolipids result from the condensation of aliphatic fatty acids (lipids) and carbohydrates. Their names are taken from the nature of the carbohydrate moiety. Consequently, glycolipids containing sophorose in the hydrophilic segment are called sophorolipids (SL); those containing rhamnose are named rhamnolipids; those with trehalose, trehalose‐lipids, and so on. Of all the glycolipid types, SL and rhamnolipids have been among the most studied [7, 8].
SL ( Figure 2.2) contain the disaccharide sophorose linked to a fatty acid via an ether function. The fatty acid must be previously hydroxylated somewhere in the carbon chain, usually at the other end of the carboxylic acid function. SL can be found as different chemical structures. The most evident and well known is the divergence between open and cyclic arrangements [9]. Open SL are those that have the chemical functionality of carboxylic acid (COOH) at the end of the lipophilic chain, while cyclic arrangements are those having an ester functionality as a result of the condensation between the fatty acid and one of the hydroxyl motifs of the sophorose.
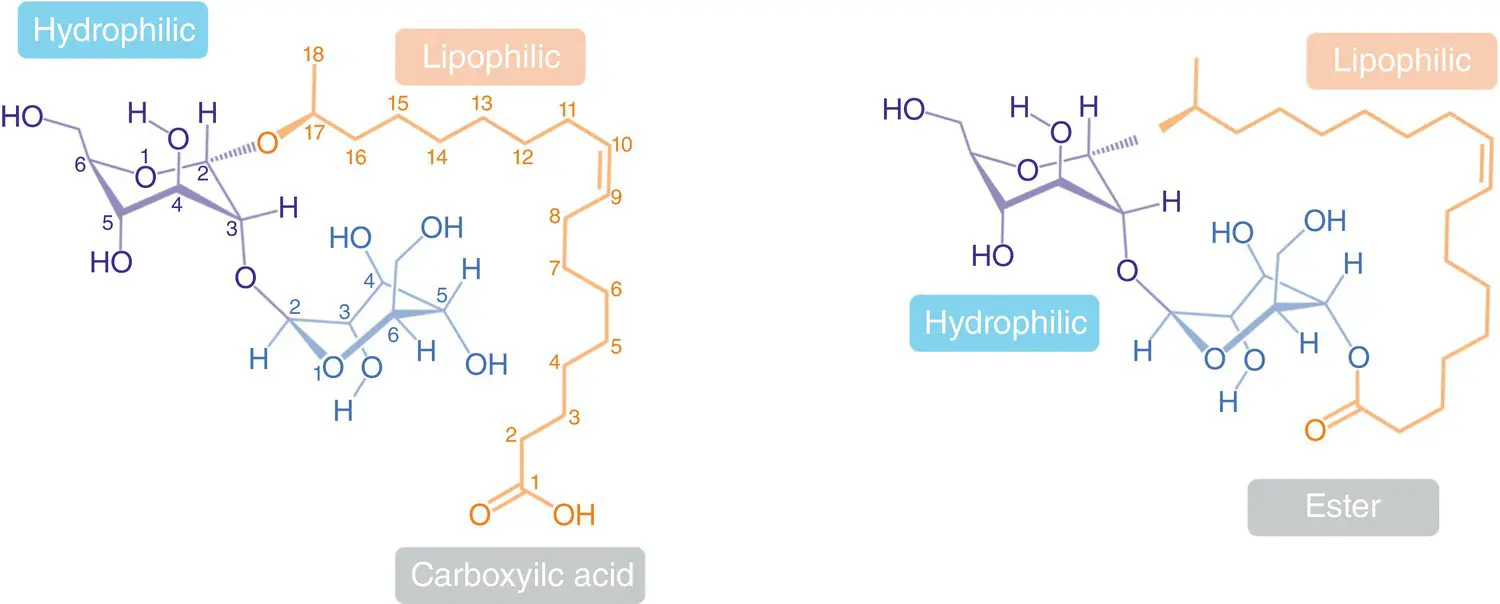
Figure 2.2 Chemical structures for two species of sophorolipids as a result of the condensation of sophorose with oleic acid. On the left side, it is shown the open form of carboxylic acid and on the right side, the cyclic form of the lactone.
Cyclic esters are called lactones. Hence, there are two types of SL: the acidic forms and the lactone forms. Other less notable molecular variables are (i) the presence or absence of acetyl groups attached to the hydroxyl moieties on the carbohydrate periphery, (ii) the length of the alkyl chain, (iii) the degree of unsaturation (unsaturation = double or triple bonds), (iv) the position of the hydroxyl group in the alkyl chain, (v) the position of the hydroxyl group of the sophorose that serves to build the ether bond with the fatty alcohol, and (vi) the position of the hydroxyl group of sophorose serves to construct the ester bond with the fatty acid in the lactone forms, inter alia.
Figure 2.2shows the chemical structures of two SL: one in the acid form (left) and one in the lactone form (right). Both species do not contain any acetyl groups but have a fatty acid moiety of 18 carbon atoms with only one unsaturation in position C‐9 with Z geometry. This fatty acid (oleic acid) must have been previously hydroxylated inside the cell by some biochemical β ‐oxidation at position C‐17. This latter allows its association with the free hydroxyl moiety of the anomeric carbon of sophorose, which produces the ether bridge of sophorose‐lipid equally found in both acid and lactone arrangements. Thus, the chemical name for the acid form should be ( S , Z )‐17‐{[(2 S ,3 R ,4 S ,5 S ,6 R )‐4,5‐dihydroxy‐6‐hydroxymethyl‐3‐{[(2 S ,3 R ,4 S ,5 S ,6 R )‐3,4,5‐trihydroxy‐6‐hydroxymethyltetrahydro‐2 H ‐pyran‐2‐yl]oxy}tetrahydro‐2 H ‐pyran‐2‐yl]oxy}octadec‐9‐enoic acid.
In the case of lactone, the carboxylic acid was condensed with the hydroxyl group in C‐5 of the other monosaccharide fragment of sophorose. After imagining the number of possible combinations of structural variations, one would not expect microorganisms to produce unique and pure compounds, but rather a wide variety of many different species. Therefore, the selection of microorganism strain, culture conditions, culture media, and substrates are the first fundamental factors playing a decisive role in the complexity (or simplicity) of obtained mixtures.
Читать дальше













