James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The chemistry of biodiesel production is known as transesterification insofar as the process involves reaction of glycoside-containing plant oil with a short chain alcohol such as methanol or ethanol. The feedstocks are typically animal, and plant fats and oils are typically made of triglycerides which are esters of free fatty acids with the trihydric alcohol, glycerol. In the transesterification reaction, the alcohol is deprotonated with a base to make it a stronger nucleophile. Commonly, ethanol or methanol is used. Usually, the reaction proceeds at a low rate; heat, as well as an acid or base, is used to help the reaction proceed more quickly.
Almost all biodiesel from waste oil is produced using the base-catalyzed technique as it is the most economical process, requiring only low temperatures and pressures and producing over 98% conversion yield (provided the starting oil is low in moisture and free fatty acids). The major steps required to synthesize biodiesel are purification, neutralization, and transesterification.
Purification: the waste vegetable oil is filtered to remove dirt, charred food, and other non-oil material often found. Water is removed because its presence causes the triglycerides to hydrolyze to give salts of the fatty acids instead of undergoing transesterification to give biodiesel. The crude oil may be stirred with a drying agent such as magnesium sulfate to remove the water in the form of water of crystallization. The drying agent can be separated by decanting or by filtration, but the viscosity of the oil may not allow the drying agent to mix thoroughly.
Neutralization of free fatty acids: A sample of the cleaned oil is titrated against a standard solution of base in order to determine the concentration of free fatty acids (RCOOH) present in the waste vegetable oil sample. The quantity of base required to neutralize the acid is then calculated.
Transesterification: While adding the base, a slight excess is factored in to provide the catalyst for the transesterification. The calculated quantity of base (usually sodium hydroxide) is added slowly to the alcohol, and it is stirred until it dissolves. Sufficient alcohol is added to make up three full equivalents of the triglyceride, and an excess is added to drive the reaction to completion. The solution of sodium hydroxide in the alcohol is then added to a warm solution of the waste oil, and the mixture is heated (typically 50°C, 122°F) for several hours (4 to 8 typically) to allow the transesterification to proceed. A condenser may be used to prevent the evaporative losses of the alcohol. Care must be taken not to create a closed system which can explode.
Workup: Once the reaction is complete, the glycerol should sink. When ethanol is used, it is reported that an emulsion often forms. This emulsion can be broken by standing, centrifugation, or the addition of a low boiling (easily removed) non-polar solvent, decanting, and distilling. The top layer, a mixture of biodiesel and alcohol, is decanted. The excess alcohol can be distilled off, or it can be extracted with water. If the latter is used, the biodiesel should be dried by distillation or with a drying agent.
See also: Biodiesel, Biodiesel Feedstocks, Biodiesel Production, Transesterification.
Biodiesel – Production and Properties
Biodiesel is a diesel-equivalent fuel derived from biological sources (such as vegetable oil) which can be used in unmodified diesel-engine vehicles. It is thus distinguished from the straight vegetable oil or waste vegetable oil used as fuels in some diesel vehicles.
Biodiesel fuel is a fuel made from the oil of certain oilseed crops such as soybean, canola, palm kernel, coconut, sunflower, safflower, corn, and a hundreds of other oil-producing crops. The oil is extracted by the use of a press and then mixed in specific proportions with other agents, which causes a chemical reaction ( Figure B-1). The results of this reaction are two products, biodiesel and soap. After a final filtration, the biodiesel is ready for use. After curing, the glycerin soap which is produced as a by-product can be used as is, or can have scented oils added before use.
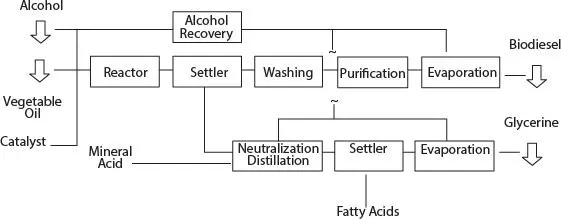
Figure B-1Biodiesel Production Path.
Biodiesel is made through a chemical process (transesterification) whereby the glycerin is separated from the fat or vegetable oil. The process leaves behind two products: (i) methyl esters and (ii) glycerin (a valuable by-product usually sold to be used in soaps and other products).
The primary products of transesterification are methyl esters (biodiesel) and glycerol. 100 lbs of soybean oil are reacted with 10 lbs of methanol and 1 lbs of catalyst yielding 100 lbs of biodiesel and 10 lbs of glycerol. Glycerol is decanted and removed first as it is heavier and sinks. Methanol is recycled in the process by washing both the glycerol and biodiesel with water to remove unreacted methanol and return it to the process. Glycerol, a valuable by-product, is approximately 88% pure and can be further processed to pharmaceutical grade glycerol.
Biodiesel is a liquid which varies in color between golden and dark brown depending on the feedstock from which it is produced. In general, biodiesel compares well to crude oil-based diesel ( Table B-5). Pure biodiesel fuel (100% esters of fatty acids) is called B100. When blended with diesel fuel, the designation indicates the amount of B100 in the blend, e.g., B20 is 20% B100 and 80% diesel, and B5 used in Europe is contains 5% B100 in diesel.
Table B-5Comparison of properties of biodiesel from various sources.
| Property | Source: waste cooking oil | Source: animal tallow | Commercial diesel fuel |
|---|---|---|---|
| Density (kg/l at 15 oC) | 0.890 -0.897 | 0.856 - 0.877 | 0.075-0.840 |
| Flash point ( oC) | 196 | 30 to 35 | 67 to 85 |
| Pour point ( oC) | 11 | -6 to 0 | -19 to -13 |
| Cetane number | 54 | 58.8 - 61 | 40-46 |
| Ash content (% w/w) | 0.004 | 0.022 – 0.025 | 0.008-0.010 |
| Sulfur content (% w/w) | 0.06 | 0.18 | 0.35-0.55 |
| Carbon residue (% w/w) | 0.33 | - | 0.35-0.40 |
| Water content (% w/w) | 0.04 | - | 0.02-0.05 |
The properties of biodiesel compare very favorably with the properties of crude oil -derived diesel. For example: (i) biodiesel is a safe, non-toxic, biodegradable, and renewable fuel that produces lower regulated vehicle emissions, (ii) biodiesel is oxygenated and has a higher flashpoint making it safer to handle and store. Biodiesel also has a higher cetane number providing a smoother running engine and extending engine life, and (iii) biodiesel can also be used in locomotives, as heating oil, in generators, and as a bio-remediator in oil spills.
Biodiesel (fatty acid methyl esters; FAME) is a notable alternative to the widely used crude oil-derived diesel fuel since it can be generated by domestic natural sources such as soybeans, rapeseeds, coconuts, and even recycled cooking oil, and thus reduces dependence on diminishing crude oil fuel from foreign sources. In addition, because biodiesel is largely made from vegetable oils, it reduces lifecycle greenhouse gas emissions by as much as 78%.
Vegetable oils and animal fats belong to an extensive family of chemicals called lipids. Lipids are bio-products from the metabolism of living creatures. As a result, they can be found widely distributed in nature. Their bio-functions are diverse, but they are most known for their energy storage capacity. Most lipids can easily dissolve in common organic solvents, meaning that they are hydrophobic. If a lipid is a solid at 25°C (77°F), it is classified as a fat; otherwise, it is oil. Typically, fats are produced by animals and oils by plants, but both are mainly made of triglyceride molecules, which are tri-esters of glycerol (a triol) and free fatty acids (long alkyl chain carboxylic acids). Other glyceride species, such as di-glycerides and mono-glycerides, are obtained from triglycerides by the substitution of one and two fatty acid moieties, respectively, with hydroxyl groups.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.