James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
In the sump underneath the scrubber, the absorbed nitrogen oxides are biologically reduced to nitrogen and ethanol is also consumed and, thus, the iron chelate solution is regenerated. The presence of oxygen and acid compounds in the flue gas, such as hydrogen chloride and hydrogen fluoride, oxidizes a part of iron chelate to the ferric (Fe 3+) state. Therefore, a purge and makeup of iron chelate is necessary to eliminate this oxidized ferric material. To minimize iron chelate consumption, a nano-filtration can be installed and bleed from the unit is passed through the filter and the chelate is recovered.
See also: THIOPAQ DeSOx Process.
Biodesulfurization
The biocatalyst desulfurization of a fuel is one of a number of possible modes of applying biologically-based processing to the needs of the renewable fuel industry. In addition, mycobacterium goodie has been found to desulfurize benzothiophene to produce α-hydroxystyrene.
Biodesulfurization is a technology to remove sulfur from the feedstock. However, several factors may limit the application of this technology. Many ancillary processes novel to crude oil refining would be needed, including a biocatalyst fermenter to regenerate the bacteria. The process is also sensitive to environmental conditions such as sterilization, temperature, and residence time of the biocatalyst. Finally, the process requires the existing hydrotreater to continue in operation to provide a lower sulfur feedstock to the unit and is more costly than conventional hydrotreating. Nevertheless, the limiting factors should not stop the investigations of the concept and work should be continued with success in mind.
See also: Refining.
Biodiesel
The term biodiesel refers to any diesel fuel substitute derived from renewable biomass. More specifically, biodiesel refers to a family of products made from vegetable oils or animal fats and alcohol, such as methanol or ethanol, called alkyl esters of fatty acids. For these to be considered as viable transportation fuels, they must meet stringent quality standards. In most contexts, unless otherwise stated, biodiesel refers to the alkyl esters produced by transesterification of vegetable oils or animal fats.
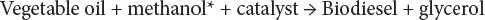
*Ethanol (ethyl alcohol, C 2H 5OH) could also be used.
Biodiesel is an alternative to crude oil-based fuels. Biodiesel is made from a variety of natural oils, especially rapeseed oil (a close cousin of canola oil) and soybean oil. Rapeseed oil tends to dominate the growing biodiesel industry in Europe, whereas in the United States, biodiesel is made from soybean oil because more soybean oil is produced here than all other sources of fats and oil combined. Palm and Jatropha oil are favored in Asian countries, but there are many other feedstock candidates, including recycled cooking oils, animal fats, and other oilseed crops.
Biodiesel is a renewable fuel that can be manufactured from algae, vegetable oils, animal fats, or recycled restaurant greases; it can be produced locally in most countries. It is safe, biodegradable, and reduces air pollutants, such as particulates, carbon monoxide, and hydrocarbon derivatives. Blends of 20% biodiesel with 80% crude oil diesel (B20) can generally be used in unmodified diesel engines. Biodiesel can also be used in its pure form (B100), but may require certain engine modifications to avoid maintenance and performance problems.
Biodiesel is made by chemically reacting vegetable oil or animal fat (or combinations of oils and fats) with alcohol (usually nearly pure methanol or ethanol) and a catalyst (sodium hydroxide). The chemical reaction converts constituents of the vegetable oil into an ester (biodiesel fuel) and glycerol. Since the biodiesel is less dense than the glycerol, it floats on top of the glycerol and may be pumped off, or the glycerol can be drained off the bottom. The fuel can then be filtered and used in heating or lighting applications. It is possible to use the product in diesel engines without further processing, but caution is advised because of the potential for engine damage and it is recommended that impurities (unreacted alcohol and sodium hydroxide) are removed by a washing.
Biodiesel is currently produced entirely from vegetable oil, waste cooking oil, animal fats, and algae. It is named biodiesel because it is derived from biological product and has similar physical properties as petro diesel. The biodiesel feedstock (oil and fats) primarily consists of triglycerides and have some amount of free fatty acids depending on their origin. The high viscosity and poor combustion properties of vegetable oils (compared to petro diesel) do not allow them to use directly as diesel substitute in engines. The direct use of vegetable oils can give rise to significant carbon deposit, lubricating oil contamination, excessive engine wear, and thus reduced engine durability. The processes such as transesterification, pyrolysis, catalytic cracking, and non-catalytic cracking have evolved to transform the vegetable oils or fats into biodiesel (fatty acid methyl ester) or biofuel.
Biodiesel is composed of long-chain fatty acids with an alcohol attached, often derived from vegetable oils. It is produced through the reaction of a vegetable oil with methyl alcohol or ethyl alcohol in the presence of a catalyst. Animal fats are another potential source. Commonly used catalysts are potassium hydroxide (KOH) or sodium hydroxide (NaOH). The chemical process is called transesterification which produces biodiesel and glycerin. Chemically, biodiesel is called a methyl ester if the alcohol used is methanol. If ethanol is used, it is called an ethyl ester. They are similar, and currently, methyl ester is cheaper due to the lower cost for methanol. Biodiesel can be used in the pure form, or blended in any amount with diesel fuel for use in compression ignition engines.
Biodiesel is typically produced through the reaction of a vegetable oil or animal fat with methanol or ethanol in the presence of a catalyst to yield glycerin and mono-alkyl esters. If methanol is used as the alcohol for transesterification, the reaction is referred to as methanolysis and methyl esters are produced, whereas ethyl esters are produced when ethanol is used for transesterification (ethanolysis). However, it is important to point out that glycerin is considered a valuable co-product that can be used in pharmaceuticals, cosmetics, and many other applications, depending on its grade and purity. The process can be represented simply as:
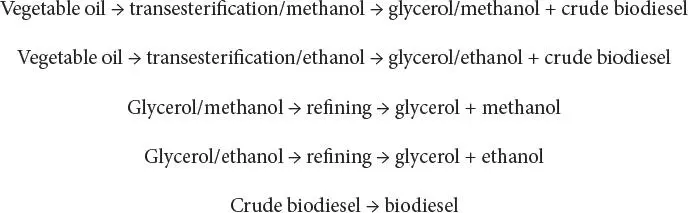
The process involves treatment of raw vegetable oils at low temperatures of 30 to 40°C (86 to 104°F) with methanol or ethanol in the presence of a catalyst such as sodium or potassium hydroxide (transesterification). Glycerol is produced and immediately settles out because of its low solubility in the ester product, carrying most of the dissolved catalyst with it. The upper layer containing the desired product is washed thoroughly to remove any residual catalyst and other emulsion-forming contaminants, yielding a clear, dark yellow material that can be directly used as a diesel substitute after dehydration. Phase separation can only be attained in ethanolysis under complete absence of water, whereas this process is often easier when methanol is used for transesterification.
Biodiesel may be made from virtually any oil-containing or oil-producing vegetation, including the seeds from rape, soybean, sunflower, peanut, cotton, castor and palm, or even animal fats. Other tropical oilseeds are also amenable to biodiesel production, such as Ricinus communis (mamona), Jatropha curcas (Jatropha), and Cyperus esculentus (tigernut). It may also be made from waste vegetable oils and fats from fish and chip or fried chicken establishments. The manufacturing process is totally benign, having no detrimental effect on the environment.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.