James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
See also: Biodiesel – Properties.
Biodiesel – Transesterification
Transesterification (alcoholysis) is the conversion of triacylglycerol lipids by alcohols to alkyl esters without first isolating the free fatty acids (May, 2004). The purpose of transesterification of vegetable oils to their methyl esters (biodiesel) process is to lower the viscosity of the oil. The transesterification reaction is affected by alcohol type, molar ratio of glycerides to alcohol, type and amount of catalyst, reaction temperature, reaction time, and free fatty acids and water content of vegetable oils or animal fats. The transesterification reaction proceeds with or without a catalyst by using primary or secondary monohydric aliphatic alcohols having 1–8 carbon atoms as follows:
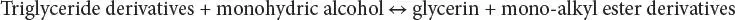
Generally, the reaction temperature near the boiling point of the alcohol is recommended. The reactions take place at low temperatures (approximately 65°C, 160°F) and at modest pressures (2 atm, 1 atm = 14.7 psi). Biodiesel is further purified by washing and evaporation to remove any remaining methanol. The oil (87%), alcohol (9%), and catalyst (1%) are the inputs in the production of biodiesel (86%), the main output. Pretreatment is not required if the reaction is carried out under high pressure (9,000 kPa) and high temperature (240°C, 465°F), where simultaneous esterification and transesterification take place with maximum yield obtained at temperatures ranging from 60 to 80°C (140 to 175°F) at a molar ratio of 6:1. The alcohols employed in the transesterification are generally short chain alcohols such as methanol, ethanol, propanol, and butanol. It was reported that when transesterification of soybean oil using methanol, ethanol, and butanol was performed, 96–98% of ester could be obtained after 1 hour.
Biodiesel – Transesterification, Catalytic
Transesterification reactions can be catalyzed by alkalis or enzymes . The catalytic transesterification of vegetable oils with methanol is an important industrial method used in biodiesel synthesis. Also known as methanolysis, this reaction is well established using acids or alkalis, such as sulfuric acid or sodium hydroxide as catalysts. However, these catalytic systems are less active or completely inactive for long chain alcohols. Usually, industries use sodium or potassium hydroxide or sodium or potassium methoxide as catalyst since they are relatively cheap and quite active for this reaction. Enzyme-catalyzed procedures, using lipase as catalyst, do not produce side reactions, but the lipases are expensive for industrial-scale production and a three-step process was required to achieve a 95% conversion. The acid-catalyzed process ( Table B-7) is useful when a high amount of free acids is present in the vegetable oil, but the reaction time is on the order of 48 to 96 hours, even at the boiling point of the alcohol, and a high molar ratio of alcohol was needed (20:1 wt/wt to the oil).
Table B-7Schematic of the catalytic transesterification process for biodiesel production.
| Feedstock | Reactor | Products |
|---|---|---|
| Vegetable oil | Catalyst | Biodiesel |
| Methanol * | Glycerol | |
| *Ethanol may also be used. |
The transesterification process is catalyzed by alkaline metal alkoxides, and hydroxides, as well as sodium or potassium carbonates. Alkali-catalyzed transesterification with short-chain alcohols, for example, generates high yields of methyl esters in short reaction times. The alkaline catalysts show high performance for obtaining vegetable oils with high quality, but a question often arises; that is, the oils contain significant amounts of free fatty acids which cannot be converted into biodiesels but to a lot of soap. These free fatty acids react with the alkaline catalyst to produce soaps that inhibit the separation of the biodiesel, glycerin, and wash water. Triglycerides are readily transesterified in a batch operation in the presence of alkaline catalyst at atmospheric pressure and at a temperature of approximately 60 to 70°C (140 to 160°F) with an excess of methanol. It often takes at least several hours to ensure the alkali (NaOH or KOH) catalytic transesterification reaction is complete. Moreover, removal of these catalysts is technically difficult and brings extra cost to the final product. Nevertheless, these methods are a good alternative since they can give the same high conversions of vegetable oils just by increasing the catalyst concentration to 1 or 2 mol%. Alkaline metal alkoxides (as CH 3ONa for the methanolysis) are the most active catalysts since they give high yields (> 98%) in short reaction times (30 min) even if they are applied at low molar concentrations (0.5 mol%).
The transesterification process is catalyzed by sulfuric, hydrochloric, and organic sulfonic acids. In general, acid-catalyzed reactions are performed at high alcohol-to-oil molar ratios, low-to-moderate temperatures and pressures, and high acid catalyst concentrations. These catalysts give high yields in alkyl esters, but these reactions are slow, requiring typically temperature above 100°C (212°F) and more than 3 h to complete the conversion. Studies of the acid-catalyzed system have been limited in number. Despite the relatively slow reaction rate, the acid-catalyzed process offers benefits with respect to its independence from free fatty acid content and the consequent absence of a pretreatment step. These advantages favor the use of the acid-catalyzed process when using waste cooking oil as the raw material.
Enzyme-catalyzed reactions (such as lipase-catalyzed reactions) have advantages over traditional chemical-catalyzed reactions: the generation of no by-products, easy product recovery, mild reaction conditions, and catalyst recycling. Also, enzymatic reactions are insensitive to free fatty acids and water content in waste cooking oil. As for the enzyme-catalyzed system, it requires a much longer reaction time than the other two systems. The enzyme reactions are highly specific and chemically clean. Because the alcohol can be inhibitory to the enzyme, a typical strategy is to feed the alcohol into the reactor in three steps of 1:1 mole ratio each. The reactions are slow, with a three-step sequence requiring from 4 to 40 hours, or more. The reaction conditions are modest, from 35 to 45°C (95 to 113°F). The main problem of the enzyme-catalyzed process is the high cost of the lipases used as catalyst.
Synthesis of biodiesel using enzymes such as Candida antarctica , Candida rugasa , Pseudomonas cepacia , immobilized lipase (Lipozyme RMIM) , Pseudomonas sp., and Rhizomucor miehei is well reported in the literature. In the previously mentioned work (Shah and Gupta, 2007), the best yield 98% (w/w) was obtained by using Pseudomonas cepacia lipase immobilized on celite at 50°C in the presence of 4–5% (w/w) water in 8 hours.
Biodiesel – Transesterification, Supercritical Methanol
The transesterification of triglycerides by supercritical methanol (SCM), ethanol, propanol, and butanol has proved to be the most promising process. Recently, a catalysts-free method was developed for biodiesel production by employing supercritical methanol (Saka and Kusdiana, 2001). The supercritical treatment at 350°C, 43 MPa, and 240 s with a molar ratio of 42 in methanol is the optimum condition for transesterification of rapeseed oil to biodiesel fuel (Kusdiana and Saka, 2004a).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.