James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Benzene (C 6H 6) is the least complex aromatic hydrocarbon, and it was the first one named as such. Each carbon atom in the hexagonal cycle has four electrons to share. One goes to the hydrogen atom, and one to each of the two neighboring carbon atoms which leaves one electron to share with one of the two neighboring carbon atoms, thus creating a double bond with one carbon and leaving a single bond with the other, which is why some representations of the benzene molecule portray it as a hexagon with alternating single and double bonds.
After benzene, aromatic hydrocarbons can be polycyclic (also called condensed aromatic systems) ( Figure A-3):
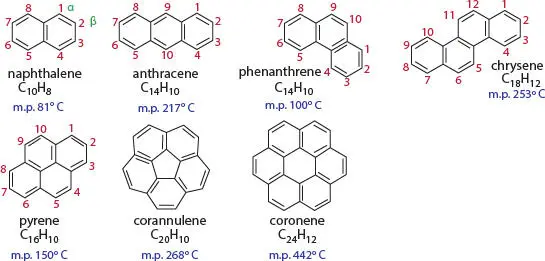
Figure A-3Examples of aromatic hydrocarbons showing the alternating double bonds and single bonds.
Other depictions of the structure portray the hexagon with a circle inside it, to indicate that the six electrons are floating around in delocalized molecular orbitals the size of the ring itself. This represents the equivalent nature of the six carbon–carbon bonds all of bond order 1.5; the equivalency is explained by resonance forms. The electrons are visualized as floating above and below the ring, with the electromagnetic fields they generate acting to keep the ring flat.
The general properties of aromatic hydrocarbons are: (i) they display aromaticity, (ii) the carbon–hydrogen ratio is high, (iii) they burn with a strong sooty yellow flame because of the high carbon–hydrogen ratio, and (iv) they undergo electrophilic substitution reactions and nucleophilic substitution reaction.
See also: Alkenes, Alicyclic Hydrocarbons, Aliphatic Hydrocarbons.
Arsenic and Selenium
Arsenic is a notoriously poisonous metalloid with many allotropic forms, including a yellow (molecular non-metallic) and several black and gray forms (metalloids). Three metalloid forms of arsenic, each with a different crystal structure, are found free in nature. However, it is more commonly found as arsenide and in arsenate compounds, several hundreds of which are known. Arsenic and its compounds are used as pesticides, herbicides, insecticides, and in various alloys.
Selenium a nonmetal, chemically related to sulfur and tellurium, which rarely occurs in its elemental state in nature. Isolated selenium occurs in several different forms, the most stable of which is a dense purplish-gray semimetal (semiconductor) form that is structurally a trigonal polymer chain. It conducts electricity and is used in photocells. Selenium is found in economic quantities in sulfide ores such as pyrite FeS 2), partially replacing the sulfur in the ore matrix. Minerals that are selenide or selenate compounds are also known, but all are rare.
Selenium salts are toxic in large amounts, but trace amounts of the element are necessary for cellular function in most, if not all, animals, forming the active center of the enzymes glutathione peroxidase and thioredoxin reductase (which indirectly reduce certain oxidized molecules in animals and some plants) and three known deiodinase enzymes (which convert one thyroid hormone to another).
Arsenic and selenium occur in biomass to the extent of several parts per million and, on combustion of the coal, a varying quantity of these elements are released or retained in the ash, depending largely on the conditions under which the combustion takes place and on the nature of the ash.
Arsenic and selenium are determined by mixing a weighed sample with the Eschka mixture followed by ignition 750°C (1,380°F). The mixture is dissolved in hydrochloric acid, and the gaseous hydride of each element is generated from the appropriate oxidation state and determined by atomic absorption spectrophotometry. The method permits measurement of the total arsenic and selenium content of coal for the purpose of evaluating these elements where they can be of concern, for example, in coal combustion. When coal samples are prepared for analysis in accordance with standard procedure, the arsenic and selenium are quantitatively retained and are representative of the total amounts in the coal
See also: Periodic Table of the Elements.
Asabe Standard X593
The American Society of Agricultural and Biological Engineers (ASABE) is an educational and scientific organization dedicated to the advancement of engineering applicable to agricultural, food, and biological systems. Founded in 1907 and headquartered in St Joseph, Michigan, ASABE comprises 9,000 members in more than 100 countries. ASABE membership is open to all (engineers as well as non-engineers) who are interested in the knowledge and application of engineering in agricultural, food, and biological systems.
ASABE Standard X593 is the standard introduced by the American Society of Agricultural and Biological Engineers (ASABE) entitled “Terminology and Definitions for Biomass Production, Harvesting and Collection, Storage, Processing, Conversion and Utilization.”
The purpose of the standard is to provide uniform terminology and definitions in the general area of biomass production and utilization. This standard includes many terminologies that are used in biomass feedstock production, harvesting, collecting, handling, storage, pre-processing and conversion, bioenergy, biopower, and bioproducts. The terminologies were reviewed by many experts from all of the different fields of biomass and bioenergy before being accepted as part of the standard.
See also: Biomass.
Ash
Ash is the noncombustible residue remaining after complete combustion of a fuel and is composed primarily of oxides and sulfates, and it should not be confused with mineral matter, which is composed of the unaltered inorganic minerals in the fuel. In very general terms, the inorganic materials in most solid fuels, including biomass, can be divided into two broad fractions: (i) inherent inorganic material and (ii) extraneous inorganic material.
The inherent inorganic material exists as part of the organic structure of the fuel, and is most commonly associated with the oxygen-, sulfur-, and nitrogen-containing functional groups. These organic functional groups can provide suitable sites for the inorganic species to be associated chemically in the form of cations or chelates. Biomass materials tend to be relatively rich in oxygen-containing functional groups, and a significant fraction of the inorganic material in some of the lower ash biomass fuels is commonly in this form. It is also possible for inorganic species to be present in fine particulate form within the organic structure of some of the fuels, and to behave essentially as an inherent component of the fuel.
The extraneous inorganic material has been added to the fuel through geological processes, or during harvesting, handling, and processing of the fuel. Biomass fuels, for instance, are commonly contaminated with soil and other materials, which have become mixed with the fuel during collection, handling and storage.
Ash is quantitatively and qualitatively different from the mineral matter originally present in the fuel because of the various changes that occur, such as loss of water from silicate minerals, loss of carbon dioxide from carbonate minerals, oxidation of iron pyrite to iron oxide, and fixation of oxides of sulfur by bases such as calcium and magnesium. In fact, incineration conditions determine the extent to which the weight changes take place and it is essential that standardized procedures be closely followed to ensure reproducibility.
Thus, ash is formed as the result of chemical changes that take place in the mineral matter during the ashing process. The quantity of ash can be more than, equal to, or less than the quantity of mineral matter in the fuel, depending on the nature of the mineral matter and the chemical changes that take place in ashing. The various changes that occur include (i) loss of water from silicate minerals, (ii) loss of carbon dioxide from carbonate minerals, (iii) oxidation of iron pyrite to iron oxide, and (iv) fixation of oxides of sulfur by bases such as calcium and magnesium. In fact, incineration conditions determine the extent to which the weight changes take place and it is essential that standardized procedures be closely followed to ensure reproducibility.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.