James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Self-purification is a result of the processes previously described above in which a chemical in the environment rapidly loses the original properties and disintegrates into various products. These products may have different a chemical composition and structure to the original chemical and exist in different migrational forms, and they undergo chemical transformations that slow after reaching thermodynamic equilibrium with the environmental parameters. Eventually, the original and intermediate compounds disappear, and carbon dioxide and water form. This form of self-purification inevitably happens in water ecosystems if the amount of toxic chemicals spilled into the system does not exceed acceptable limits.
While acid-base reactions are not the only chemical reactions important in aquatic systems, they do present a valuable starting point for understanding the basic concepts of chemical equilibria in such systems. Carbon dioxide (CO 2), a gaseous inorganic chemical substance of vital importance to a variety of environmental processes, including growth and decomposition of biological systems, climate regulation, and mineral weathering, has acid-base properties that are critical to an understanding of its chemical behavior in the environment. The phenomenon of acid rain is another example of the importance of acid-base equilibria in natural aquatic systems.
Furthermore, the almost unique physical and chemical properties of water as a solvent are of fundamental concern to aquatic chemical processes. For example, in the liquid state, water has unusually high boiling point and melting point temperatures compared to its hydride analogues from the periodic table of the elements (Table 8.10) such as ammonia (NH 3), hydrogen fluoride (HF), and hydrogen sulfide (H 2S). Hydrogen bonding between water molecules means that there are strong intermolecular forces making it relatively difficult to melt or vaporize.
In addition, pure water has a maximum density at 4°C (39°F), higher in temperature than its freezing point (0°C, 32°F), and that ice is substantially less dense than liquid water, and is important (and fortunate) in several contexts. Thus, as water in a lake is cooled at its surface by loss of heat to the atmosphere, the ice structures formed will float. Furthermore, a dynamically stable water layer near 4°C (39°F), will tend to accumulate at the bottom of the lake, and the overlying, less dense water able to continue cooling down to the freezing point. This means that ice will eventually coalesce at the surface, forming an insulating layer that greatly reduces the rate of freezing of the underlying water. This situation is obviously important for plants and animals that inhabit lake waters.
In addition, the presence of salt components means that the temperature of maximum density for seawater is shifted to lower temperatures: in fact, the density of seawater continues to increase right down to the freezing point. The high concentration of electrolytes in seawater assist in breaking up the open, hydrogen-bonded ice-like structure of water near its freezing point. Because the salt components tend to be excluded from the ice formed by freezing seawater, sea ice is relatively fresh and still floats on water. Much of the salt it contains is not truly part of the ice structure but contained in brines that are physically entrained by small pockets and fissures in the ice.
The dielectric constant of water (78.2 at 25°C, 77°F) is high compared to most liquids. Of the common liquids, few have comparable values at this temperature, e.g., hydrogen cyanide (HCN, 106.8), hydrogen fluoride (HF, 83.6), and sulfuric acid (H 2SO 4, 101). By contrast, most non-polar liquids have dielectric constants on the order of 2. The high dielectric constant helps liquid water to solvate ions, making it a good solvent for ionic substances, and arises because of the polar nature of the water molecule and the tetrahedrally-coordinated structure in the liquid phase.
In many electrolyte solutions of interest, the presence of ions can alter the nature of the water structure. Ions tend to orient water molecules that are near to them. For example, cations attract the negative oxygen end of the water dipole toward them. This reorientation tends to disrupt the ice-like structure further away. This can be seen by comparing the entropy change on transferring ions from the gas phase to water with a similar species that does not form ions.
As an example or chemical transformation that can occur in a water system, the chemistry of methyl iodide (which is thermodynamically unstable in seawater) is known and its chemical fate is kinetically controlled. The equations showing the fate of methyl iodide are as follows:
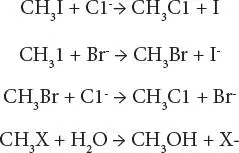
In this equation, X = C1 -, Br -, I -
Chloride ion was theoretically predicted to be the most kinetically reactive species, with water second, and other anions of lesser importance. This suggested that methyl iodide in seawater would react predominantly via a nucleophilic substitution reaction with chloride ion to yield methyl chloride. Methyl iodide and the methyl chloride produced by would also react with water, although more slowly, to yield methanol and halide ions. According to these experiments, substantial amounts of methyl chloride should be formed in seawater. Methyl chloride has a long half-life for decomposition by known reactions in seawater. Hence, its presence could be a useful label for some surface-derived water masses. Methyl chloride is in fact found in the atmosphere, where compared to methyl iodide, it is less stable to photo-degradation reactions.
Steroids are a class of biogenic compounds which may serve as an indicator of certain processes transforming matter in seawater and sediments. The steroid hydrocarbon structure ( Figure A-2) forms a relatively stable nucleus which may incorporate functional groups such as alcohols (sterol derivatives and stanol derivatives), ketone derivatives (stanone derivatives) and olefin linkages (sterene derivatives) either in the four ring system or on the side chain originating at C-17.
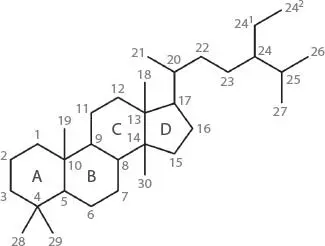
Figure A-2The hydrocarbon framework of the steroid system (ring lettering and atom numbering are shown).
These compounds are produced by a wide variety of marine and terrestrial organisms and often have specific species sources. Diagenetic alteration of steroids by geochemical and biochemical processes can lead to the accumulation of transformed products in seawater and sediments.
Within the group of chlorinated compounds, chlorinated ethylene derivatives are the most often detected groundwater pollutants. Tetrachloroethylene (PCE) is the only chlorinated ethylene derivative that resists aerobic biodegradation. Trichloroethylene (TCE), all three isomers of dichloroethylene (CCl 2= CH2and the cis/trans isomers of CHCl=CHCl), and vinyl chloride (CH 2=CHC1) are mineralized in aerobic co-metabolic processes by methanotropic or phenol-oxidizing bacteria. Oxygenase derivatives with broad substrate spectra are responsible for the co-metabolic oxidation. Vinyl chloride is furthermore utilized by certain bacteria as carbon and electron source for growth. All chlorinated ethylene derivatives are reductively dechlorinated under anaerobic conditions with possibly ethylene or ethane as harmless end-products.
Tetrachloroethylene (CCl 2=CCl 2) is dechlorinated to trichloroethylene (CCl 2=CHCl) in a co-metabolic process by methanogens, sulfate reducers, homoacetogen derivatives, and others. Furthermore, tetrachloroethylene and trichloroethylene serve in several bacteria as terminal electron acceptors in a respiration process. The majority of these isolates dechlorinate tetrachloroethylene and trichloroethylene to cis-l,2-dichloroethene, although they have been isolated from systems where complete dechlorination to ethene occurred.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.