James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The most commonly applied technique for the determination of the ash content and ash composition of coals and other solid fuels in the laboratory involves heating the fuel slowly in air to constant mass at a temperature of 815°C (1,500°F), and subjecting the resultant ash residue to chemical elemental analysis. The ash residue is normally weighed to provide an estimate of the ash content of the fuel, and then analyzed for the 10 major elements present in coal ashes, i.e. Si, Al, Fe, Ca, Mg, Ti, Na, K, P, and S. The elemental concentrations are conventionally expressed as oxides, in their highest oxidation states. The analysis of the laboratory-prepared ash for its trace element content is also fairly common practice. This is a perfectly reasonable and practical approach for most coals, and many other solid fuels, and has been applied for biomass ash analysis.
See also Ash, Ash Composition.
As-Received Moisture
The as-received moisture of a carbonaceous samples is the moisture present in a sample when delivered to the analytical laboratory or to the customer. Moisture content can affect the self-heating rate of the sample in two ways: changing the overall heat balance and the rate of the oxidation reaction.
It has been suggested that there is a critical moisture content at which the rate of oxidation reached a maximum, but the reaction may be subject to the means of conducting the test which can change the subsequent low-temperature oxidation behavior of the sample.
Atmosphere
The atmosphere is the envelope of gases surrounding the earth, and it is subdivided into regions depending on the altitude. The constituents of the atmosphere are primarily nitrogen (N 2, 78.08% v/v), oxygen (O 2, 20.95% w/w), and water vapor (0 to 0.25% w/w), although the concentration of water vapor (H 2O) is highly variable, especially near the surface, where volume fractions can be as high as 4% in the tropics. There are many minor constituents or trace gases such as (alphabetically rather than by abundance, which is variable) argon (Ar), carbon dioxide (CO 2), helium (He), hydrogen (H 2), krypton (Kr), methane (CH 4). (neon, Ne), nitrous oxide (N 2O), ozone (O 3), and water vapor (H 2O) ( Table A-26).
Table A-26Approximate Composition of the Atmosphere.
| Component, % v/v | Amount, % v/v |
|---|---|
| Major components: | |
| Nitrogen (N 2) | 78.08 |
| Oxygen (O 2) | 20.95 |
| Minor/trace components: | |
| Argon (Ar) | 0.93 |
| Water vapor (H 2O) | 0.025 * |
| Carbon dioxide (CO 2) | 0.035 |
| Neon (Ne) | 0.018 |
| Helium (He) | 0.00052 |
| Methane (CH 4) | 0.00014 |
| Krypton (Kr) | 0.00010 |
| Nitrous oxide (N 2O) | 0.00005 |
| Hydrogen (H 2) | 0.00005 |
| Ozone (O 3) | 0.0000007 |
| *Variable; can be as high as 4% v/v in humid areas. |
In addition to the gaseous constituents, the atmosphere also contains suspended solid and liquid particles. Aerosols are particulate matter usually less than 1 micron in diameter (also called 1 µm which is equivalent to 1 m x 10 -6in diameter, i.e., one millionth of a meter or one thousandth of a millimeter, 0.001 mm, or approximately 0.000039 of an inch) that are created by gas-to-particle reactions and are lifted from the surface by the winds. A portion of these aerosols can become centers of condensation or deposition in the growth of water and ice clouds. Cloud droplets and ice crystals are made primarily of water with some trace amounts of particles and dissolved gases. Their diameters range up to 100 µm. Water or ice particles larger than approximately 100 microns begin to fall because of gravity and may result in precipitation at the surface.
Ozone is found in trace quantities throughout the atmosphere, the largest concentrations being location in a layer in the lower stratosphere between the altitudes of 9 and 18 mi (15 and 30 km). This ozone results from the dissociation by solar ultraviolet radiation of molecular oxygen in the upper atmosphere and nitrogen dioxide in the lower atmosphere. Ozone also plays an important role in the formation of photochemical smog and in the purging of trace species from the lower atmosphere.
The chemistry of ozone formation can be explained in relatively simple terms, although the reactions are believed to be much more complex. Thus, above approximately 19 mi (30 km), oxygen is dissociated during the daytime by energy (hv) from ultraviolet light:

The oxygen atoms produced then form ozone:

In this equation, M is an arbitrary molecule required to conserve energy and momentum in the reaction that produces ozone. Although present in only trace quantities ( Table A-26), atmospheric ozone plays a critical role for the biosphere by absorbing the ultraviolet radiation with a wavelength from 240 to 320 nm (nm, 1 nm = 1 meter x 10 9, which would otherwise be transmitted to the surface of the Earth.
The atmospheric ozone should not be confused with the ozone layer which acts is a region of stratosphere of the Earth that absorbs most of the ultraviolet radiation from the Sun. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 9 to 22 mi above the Earth, although the thickness of the layer varies seasonally and geographically. This layer is so-named because it contains a high concentration of ozone (O 3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains up to 10 parts per million of ozone, while the average ozone concentration in the atmosphere of the Earth as a whole is on the order of 0.3 parts per million.
The ultraviolet radiation is lethal to simple unicellular organisms (algae, bacteria, protozoa) and to the surface cells of higher plants and animals. It also damages the genetic material of cells (deoxyribonucleic acid, DNA) and is responsible for sunburn in human skin. In addition, the incidence of skin cancer has been statistically correlated with the observed surface intensities of the ultraviolet wavelengths from 290 to 320 nm, which are not totally absorbed by the ozone layer.
Atmospheric pressure decreases as an approximately exponential function of altitude, which largely determines the characteristics of the atmosphere. Thus:
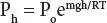
In this equation, P his the pressure at any given height, P ois the pressure at zero altitude (sea level); m is the average gram molecular mass of air (28.97 g/mole in the troposphere); g is the acceleration of gravity (981 cm x sec -1at sea level); h is the altitude (in cm or meters or kilometers), and R is the gas constant (8.314 x 107 erg x deg -1x mole -1), and T is the absolute temperature. Furthermore:

At sea level where the pressure is 1 atm:

The characteristics of the atmosphere vary widely with altitude, time (season), location (latitude), and even solar activity. At a high altitude, normally reactive species, such as atomic oxygen, persist for long periods of time. At such altitudes, the pressure is low and the distance traveled by a reactive species before it collides with a potential reactant (the mean free path) is high.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.