The conversion of HMF provides FDC and FDCA with basic and acidic attributes through photocatalytic valorization under ultraviolet, visible, and natural solar lights. Moreover, glycerol, methanol, ethanol, and toluene are converted into hydrogen and other chemical products under specific operating conditions via photocatalytic reforming.
1.3.2 TiO 2as a Significant Photocatalyst
The selection of suitable photocatalyst depends on the compatibility between band position of oxidation and reduction potentials of the reactants in a reaction. There are two types of band positions: conduction band position and valence band position. To carry out reduction reactions with electrons from the conduction band of photocatalyst, the position of conduction band should have more negative value than the reduction potential of reagents. In opposite of this, for the occurrence of oxidation reactions in photocatalysis with holes, the position of valence band should have more positive value than the oxidation potential of the reagents. The search of optimal photocatalysts for the photocatalytic reactions is based on band gap which initiate redox reactions with over potentials. TiO 2is the more chosen photocatalyst by researchers because it is non-toxic, stable, biological and chemically inert [5, 14, 15]. TiO 2is a very active photocatalyst under ultraviolet and visible light radiation especially for the conversion of biomass substrates. UV-photocatalysts can be divided into oxide (Ti 4+, Sn 4+, Ge 4+, Zr 4+, etc.) and non oxide groups. Many UV active photocatalysts can be improved by increasing the range of visible light so that more absorption of light can be done. Nowadays, the development of new catalysts for maximum absorption of visible light is in great attention with mixed metal oxides, sulfides, and nitrides for biomass utilization. Table 1.3provides a list of important homogeneous and heterogeneous catalysts as follows in which mostly heterogeneous catalysts are associated with TiO 2.
1.3.3 Factors Affecting Photocatalytic Efficiency
There are so many operation parameters in photocatalytic process which are listed below in brevity [16].
Table 1.3 List of important photocatalysts for biomass conversion [14, 15].
| Homogeneous photocatalysts |
| S. no. |
Name of catalysts |
| 1. |
Vanadium complexes |
| 2. |
[Ir(ppy) 2-(dtbbpy)]PF 6 |
| 3. |
[Ir(dF(CF 3)ppy] 2(dtbbpy)]PF 6/Na 2S 2O 8/Pd(OAc) 2 |
| 4. |
[PMim][NTf2][PrSO 3HMim][OTf] |
| 5. |
N-hydroxyphthalimide(NHPI) |
| Heterogeneous photocatalysts |
| 1. |
Pb/ZnIn 2S 4, TiO 2 |
| 2. |
TiO 2 |
| 3. |
In 2S 3 |
| 4. |
CdS/CdO x |
| 5. |
Pt/ZnS-ZnIn 2S 4 |
| 6. |
Mesoporous graphitic nitride |
| 7. |
Carbazolic copolymers |
| 8. |
Ligand-controlled CdS Quantum Dots |
| 9. |
Ir(ppy) 2(bpy)-MCFs |
| 10. |
CuO x/CeO 2/A-NTs |
| 11. |
g-C 3N 4 |
| 12. |
Au-HYT |
| 13. |
Ag-P25 |
| 14. |
Ru-LaFeO 3/Fe 2O 3 |
| 15. |
NiO/NaTaO 3 |
| 16. |
CoPz/g-C 3N 4 |
| 17. |
Nb 2O 5 |
| 18. |
WO 3/g-C 3N 4 |
| 19. |
Sn-Beta zeolite |
| 20. |
Acid hydrolysate |
| 21. |
TiO 2/Pt |
| 22. |
La/TiO 2 |
| 23. |
Pt-F-TiO 2 |
| 24. |
Cr/TiO 2/zeolite |
| 25. |
Rh/TiO 2 |
| 26. |
Heterostructured CdS/MoS 2 |
| 27. |
Ru-LaFeO 3/Fe 2O 3 |
| 28. |
Pt/Al 2O 3and Pd/Al 2O 3 |
| 29. |
ZnO-ZnS/graphene |
| 30. |
Sn-Beta zeolite |
1 1. Light intensity: Intensity of incident solar radiation is an important parameter in any photocatalytic reaction because the rate of reaction is totally dependent on the absorption of the photocatalyst so that the degradation rate can be increased with the increase in light intensity.
2 2. Nature of the photocatalyst: Nature of photocatalyst should be adaptable to oxidation reactions. For the absorption of light radiation, the different morphologies of photocatalysts are required for higher activity and stability.
3 3. Concentration of the substrate: The concentration of a substrate is an essential parameter which is going to be of use in any photocatalytic reaction. Purity and adequate concentration is required to perform such reactions to avoid the wastage of materials.
4 4. Concentration of photocatalysts: Normally, optimum concentration of photocatalyst is decided after necessary enumeration to avoid the excess of the catalyst so that absorption of enough photons can be possible to carry out a successful photochemical reaction.
5 5. pH: The knowledge about the effect of pH in any photocatalytic reaction is beneficial to carry out the photochemical reaction because pH controls the surface charge properties of the photocatalysts.
6 6. Reaction temperature: Temperature plays an important role in the reaction rate of a photocatalytic reaction for the conversion of organic substances. It is evident from the literature that photocatalytic activity is directly affected by the reaction temperature because the increase in temperature raises the chances of recombination of charge carriers and desorption of adsorbed materials which inturn decrease in the photocatalytic activity.
1.3.4 Characterization Tests
Semiconductors are main materials to generate holes and electrons after incident radiation in heterogeneous photocatalysts. To detail the study about semiconductors it is essential to check the capability of photocatalysts. There are so many characterization tests to check the possibility for utilization of biomass by photocatalysis which are summarized below in Table 1.4. In this table, all possible characterization tests are based on elemental composition, band structures, physical and chemical properties.
Figure 1.4provides a glimpse of strategy for the fabrication of photocatalysts which is given below [15].
There are some requirements to fulfill these strageies for the fabrication of any photocatalyst [15]:
1 Enable the catalysts to absorb maximum solar spectrum,
2 Separate or prevent the photoexcited charges,
3 Making tuning band gap to get enough energy for initiating the required reactions,
4 Modification in catalyst to get better stability,
5 Making catalyst to reusable,
6 Minimizing photocatalyst cost.
Table 1.4 Essential characterization tests on semiconductors used in photocatalysis [17].
| S. no. |
Characterization tests |
| 1. |
Elemental composition |
| (i) |
Chemical composition |
| (a) |
Energy dispersive X-ray spectroscopy |
| (b) |
Electron energy-loss spectroscopy |
| (c) |
High-angle annular dark-field imaging |
| (ii) |
Chemical state and structure |
| (a) |
X-ray photoelectron spectroscopy |
| (b) |
X-ray absorption spectroscopy |
| (c) |
Fourier transform infrared spectroscopy |
| (d) |
Raman spectroscopy |
| 2. |
Physical properties |
| (i) |
Physical structure |
| (a) |
Electron microscopy |
| (b) |
Atomic force microscopy |
| (c) |
Gas adsorption–desorption analysis |
| (ii) |
Crystallographic properties |
| (a) |
X-ray diffraction |
| (b) |
Transmission electron microscopy |
| (iii) |
Optical absorption |
| (a) |
Diffuse reflectance spectroscopy |
| (b) |
Finite-difference time-domain method |
| (iv) |
Charge dynamics |
| (a) |
Photoluminescence spectroscopy |
| (b) |
Transient absorption spectroscopy |
| (c) |
Surface photovoltage and photocurrent spectroscopy |
| (d) |
Electrochemical impedance spectroscopy |
| (v) |
Defects |
| (a) |
Electron spin resonance |
| (b) |
Positron annihilation spectroscopy |
| (c) |
XPS valence-band spectrum |
| (vi) |
Colloidal stability |
| (a) |
ζ- potential |
| (vii) |
Thermal stability |
| (a) |
Thermo gravimetric analysis |
| 3. |
Band structure |
| (i) |
Band gap |
| (a) |
Tauc plot |
| (b) |
Photoluminescence and surface photovoltage spectroscopy |
| (ii) |
Band edges and band edge offsets |
| (a) |
Density functional theory |
| (b) |
XPS spectrum |
| (c) |
Ultraviolet photoelectron spectroscopy |
| (iii) |
Fermi level (work function and flat-band potential) |
| (a) |
Kelvin probe force microscopy |
| (b) |
Secondary electron cutoff |
| (c) |
Photoelectrochemical methods |
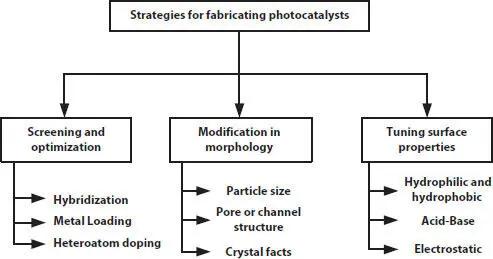
Figure 1.4 Strategies for the fabrication of photocatalysts [15].
Читать дальше













