Joseph J. Torres - Life in the Open Ocean
Здесь есть возможность читать онлайн «Joseph J. Torres - Life in the Open Ocean» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Life in the Open Ocean
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Life in the Open Ocean: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Life in the Open Ocean»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Life in the Open Ocean: The Biology of Pelagic Species
Life in the Open Ocean: The Biology of Pelagic Species
Life in the Open Ocean — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Life in the Open Ocean», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
At what concentration does oxygen become limiting in oxygen minima? A good indication is a reduction in the biomass or the diversity of the fauna inhabiting them. Childress and Seibel (1998) observed that oxygen has little influence on the biomass or species composition of midwater organisms inhabiting minima down to a level of 0.20 ml l −1or 0.63 kPa. At oxygen concentrations of 0.15 ml l −1and below, such as that in the Eastern Tropical Pacific or Arabian Sea, oxygen minimum zones (OMZ’s) exhibit reduced biomass and diversity. In those cases, anaerobiosis while resident in the minimum accompanied by a vertical migration out of the layer to more highly oxygenated waters at night is the most likely strategy (Childress and Seibel 1998).
Intertidal species, including those that dwell in burrows as well as epifaunal species such as bivalves that are exposed for extended periods, are usually highly competent anaerobes (Hochachka 1980). Their situation as intertidal dwellers is fundamentally different from that of oxygen‐minimum‐layer species. Their anoxia and normoxia are cyclic, varying with tidal exposure. Thus, it is adaptive to be able to extract oxygen efficiently, but only down to the point where a large investment in the systems involved in oxygen uptake and transport is unnecessary. For most species, that point lies in the partial pressure range of 20–30 mm Hg oxygen or 2.7–4.0 kPa (Torres et al. 1979, 1994; Childress and Seibel 1998). For intertidal species, oxygen availability will continue to decline to or near zero oxygen as the tide recedes and anaerobiosis inevitably will become necessary.
In the deep sea, it is the stability of oxygen minima in concert with the limited food resources that have allowed the highly efficient respiratory systems of oxygen‐minimum‐layer fauna to arise.
Adaptations to Oxygen Minima
The Aerobic Strategy
Species that are able to maintain an aerobic existence in oxygen minima do so by having a highly effective system for removing oxygen from seawater, allowing them to consume oxygen in sufficient quantities to sustain life at very low oxygen concentrations. The ability to regulate oxygen consumption down to very low levels of external oxygen is defined in physiological terms as having a low critical oxygen partial pressure, or P c. Figure 2.22shows the relationship of oxygen consumption rate and external PO 2for an oxygen‐minimum‐layer species, the shrimp‐like lophogastrid Gnathophausia (now Neognathophausia) ingens . The species is able to regulate its oxygen consumption (VO 2) down to the lowest oxygen level it experiences in its environment in the oxygen minimum layer off the coast of California (8 mm Hg oxygen or 0.8 kPa). The P cis the point on the curves where the oxygen consumption declines precipitously toward 0 and it varies with activity level as shown. A study on the relationship of species’ critical oxygen partial pressures vs. their minimum environmental PO 2shows that for most oxygen minimum dwellers, species’ P cs are equivalent to the lowest oxygen concentrations encountered in nature (Childress and Seibel 1998). What is surprising is that all pelagic species living in normoxic waters that have been examined can also regulate their oxygen consumption down to a low PO 2: 4–6 kPa, or 20–30% of air saturation (Childress 1975; Donnelly and Torres 1988; Cowles et al. 1991; Torres et al. 1994). Thus, even at very much higher habitat O 2levels, pelagic species maintain a P cnear 4 kPa. It is tempting to conclude that 4 kPa reflects a global ocean oxygen minimum that had to be accommodated by most pelagic species at some point during geological history. While possible, evidence does not support it (Childress and Seibel 1998). What is more likely is that the occasional high oxygen demands of increased activity and the respiratory machinery it requires coincidentally equip most species with the ability to take up and transport oxygen down to 4 kPa.
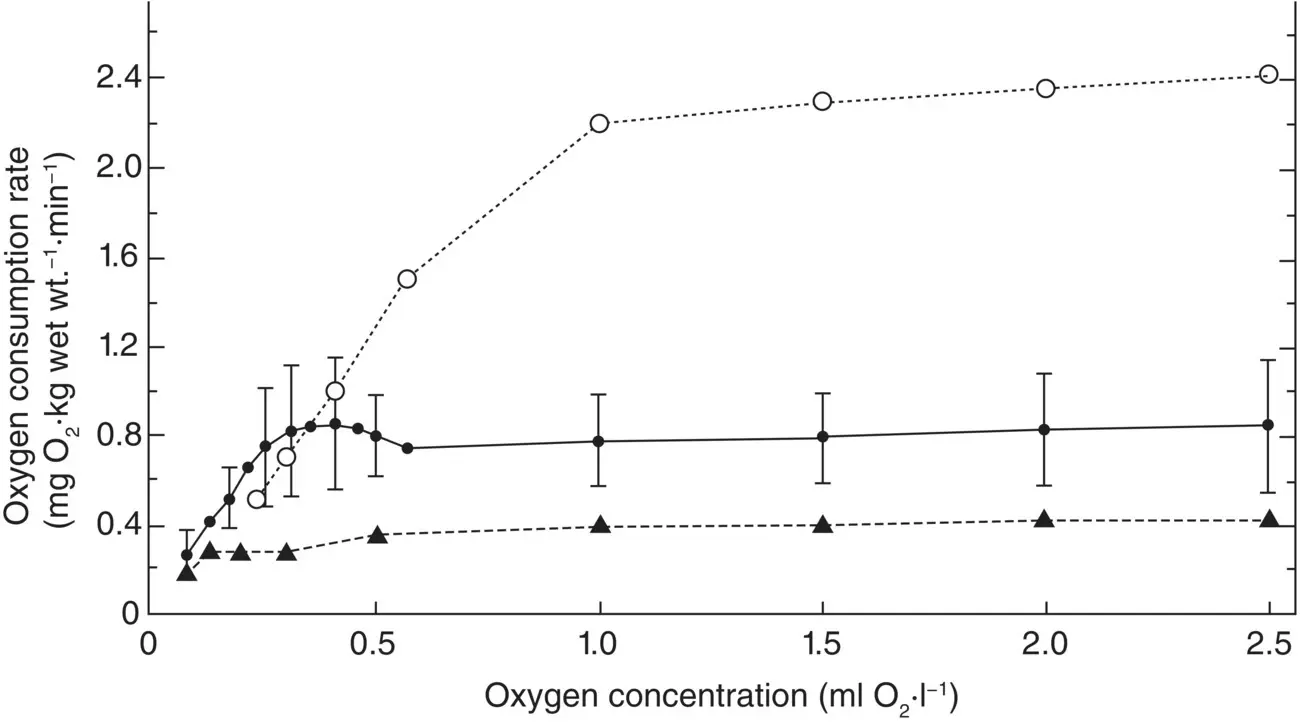
Figure 2.22 Oxygen consumption rate of the lophogastrid Gnathophausia ingens as a function of oxygen concentration (in milliliters of oxygen per liter). Open circles represent a single very active animal; closed circles represent the mean of 23 runs with 8 individuals; closed triangles represent a single non‐swimming animal. The vertical lines represent plus or minus one standard deviation.
Source: J. J. Childress, Oxygen minimum layer: vertical distribution and respiration of the mysid Gnathophausia ingens, Science , 1968, Vol 160, Issue 3833, figure 1 (p. 1242). Reprinted with permission from AAAS.
Gnathophausia ingens has been the subject of several detailed studies by Childress and his students. Their studies paint a very complete picture of how the species copes with low oxygen. Rather than any exotic adaptations, such as unusual new respiratory structures, Gnathophausia has achieved its ability to thrive in the OMZ by very highly developed gas‐exchange and circulatory systems. Six major adaptations have been noted. First, G. ingens has a very high ventilation volume; that is, it can move a considerable amount of water over its gills per unit time: up to 81 kg min −1( Figures 2.23and 2.24). Second, it is very efficient at removing oxygen from the ventilatory stream, even when that stream is moving very rapidly over the gills. This property is known as the % utilization of oxygen and in Gnathophausia it can be as high as 90% ( Figures 2.23and 2.24). That high % utilization is achieved through its third, fourth, and fifth adaptations: a very high gill surface area to maximize the possibility for exchange (9–14 cm 2g −1wet mass); a minimal diffusion distance in the gill filaments themselves (1.5–2.5 μm) to minimize the barrier for oxygen diffusion into the blood (very unusual for Crustacea); and a very effective blood pigment (hemocyanin) capable of taking up oxygen at very low concentrations (50% saturated at 0.19 kPa). A well‐developed circulatory system rounds out the suite of adaptations with the capability of delivering 225 ml kg −1min −1.
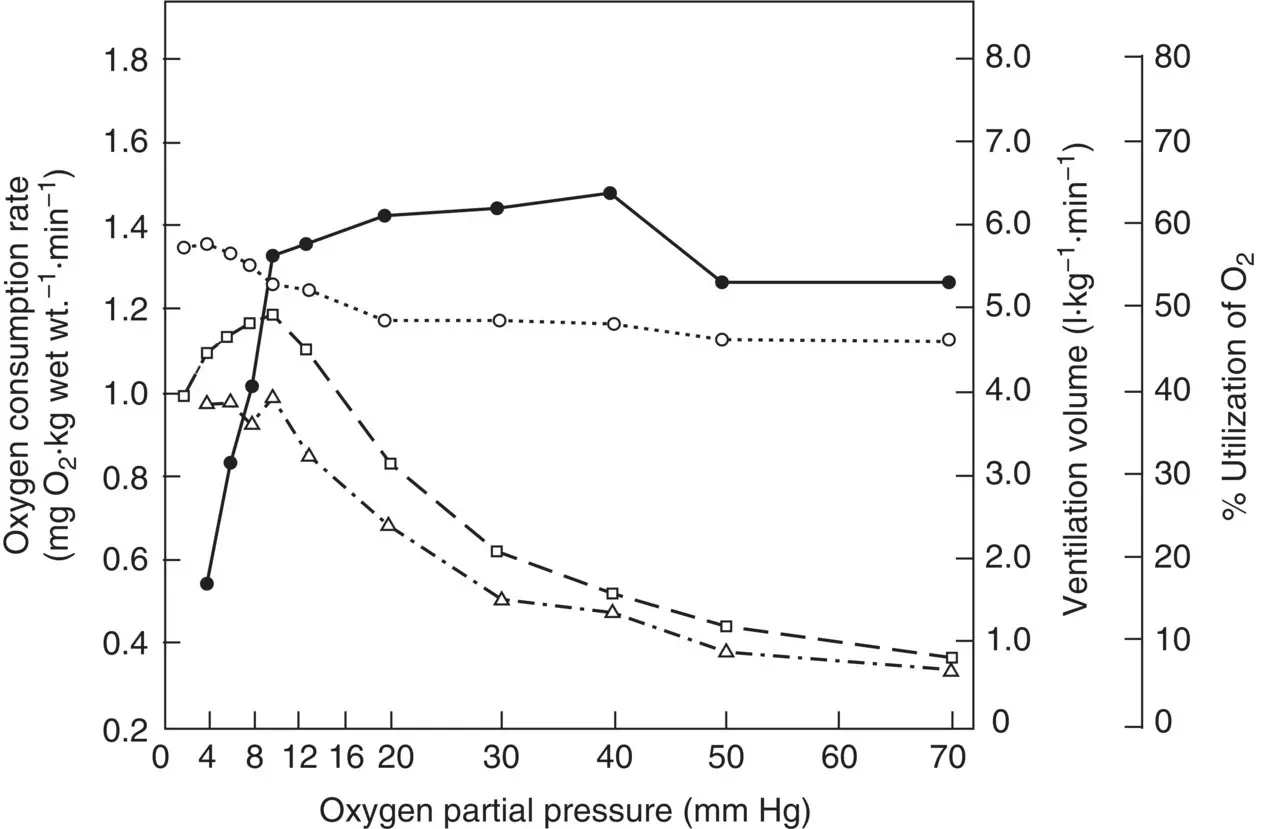
Figure 2.23 Oxygen consumption rate, percent utilization of oxygen, and ventilation volume in Gnathophausia ingens as a function of oxygen partial pressure, mean of eight runs. Solid line represents oxygen consumption rate; dotted line represents % utilization; dashed line depicts measured ventilation volume; dot‐dash line is calculated ventilation volume.
Source: Figure 4 from Childress (1971), Biol. Bull . 141: 114. Reprinted with permission from the Marine Biological Laboratory, Woods Hole, MA.
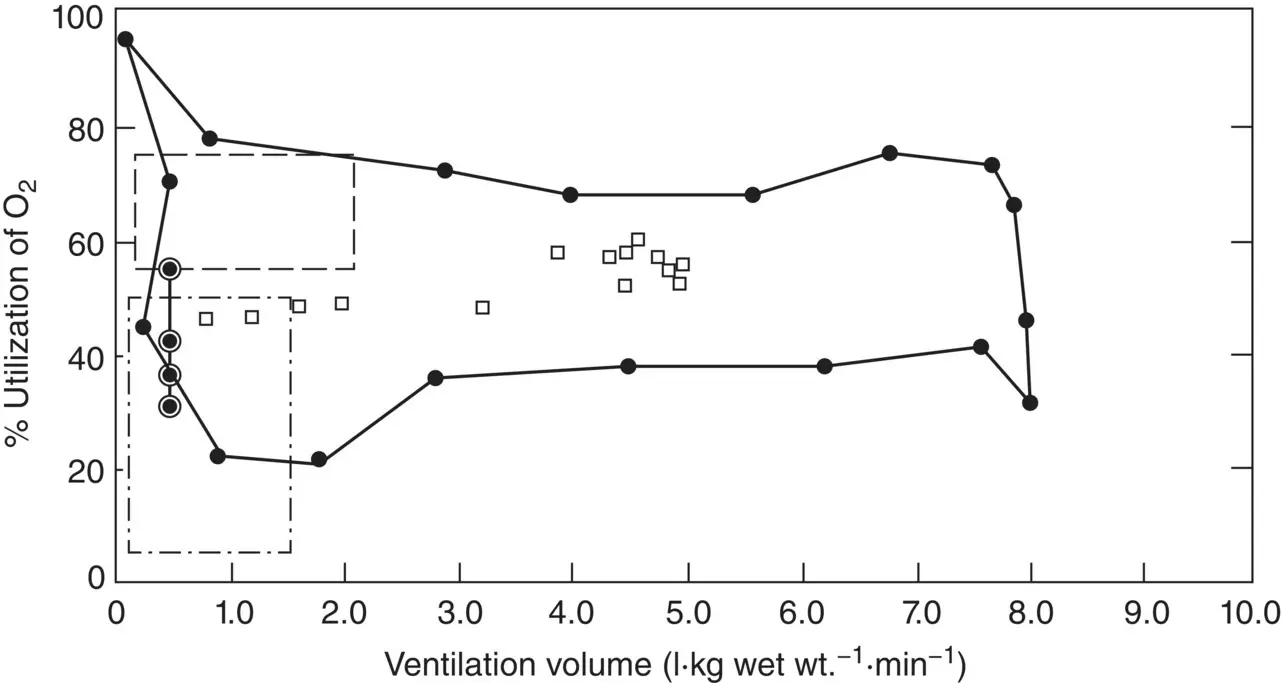
Figure 2.24 Relationship between percent utilization and ventilation volume in Gnathophausia ingens utilizing the values given in Figure 2.23.
Source: Figure 5 from Childress (1971), Biol. Bull . 141: 115. Reprinted with permission from the Marine Biological Laboratory, Woods Hole, MA.
No other oxygen‐minimum‐layer species has been examined as well as G. ingens . Taken together, the several studies on the species’ respiratory physiology paint a complete picture of how it is possible to survive at vanishingly low oxygen concentrations. Nonetheless, a few pieces of the puzzle have been collected in other taxa to suggest that elements of the suite have been employed by other species to achieve the same end. The most important characteristic to look for is a P cat or below the lowest PO 2encountered in nature. That characteristic has been observed in many of the Crustacea living in the oxygen minimum in the California borderland (8 mm Hg, Childress 1975; Childress and Seibel 1998). It has also been seen in at least one crustacean dwelling in the Eastern Tropical Pacific where the oxygen minimum layer is as low as 3 mm Hg O 2: the galatheid red crab Pleuroncodes planipes with a P cof 3 mm Hg (Quetin and Childress 1976).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Life in the Open Ocean»
Представляем Вашему вниманию похожие книги на «Life in the Open Ocean» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Life in the Open Ocean» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












