Cheanyeh Cheng - Enzyme-Based Organic Synthesis
Здесь есть возможность читать онлайн «Cheanyeh Cheng - Enzyme-Based Organic Synthesis» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Enzyme-Based Organic Synthesis
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Enzyme-Based Organic Synthesis: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Enzyme-Based Organic Synthesis»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
An insightful exploration of an increasingly popular technique in organic chemistry Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Enzyme-Based Organic Synthesis», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Since long‐chain alkanes are more persistent in the environment than shorter alkanes, the degradation of long‐chain alkane is an important topic in synthetic chemistry for dealing with the crude oil contamination in environment. Enzymes used for the activation of alkanes with long chain length (≥C 16) by hydroxylation have been found including yeast P450s [42], integral‐membrane monooxygenases (AlkM) [43], flavin‐containing alkane monooxygenases (LadA) [44], and dioxygenases [45]. It was acknowledged that thermophilic bacillus Geobacillus thermodenitrificans NG80‐2 degrades the long‐chain alkane (C 15–C 36) by utilizing a terminal oxidation pathway to convert long‐chain alkanes to their corresponding primary alcohols ( Scheme 2.8). It is also found that LadA is the key initiating enzyme in the terminal oxidation pathway of G. thermodenitrificans NG80‐2 [46]. However, studies on the bacterial strain Acinetobacter venetianus 6A2 show that this bacterial strain can utilize n ‐alkanes with chain lengths ranging from decane (C 10H 22) to tetracontane (C 40H 82) [47]. Two genes of the AlkB‐type alkane hydroxylase homologous, alkMa and alkMb , isolated from A. venetianus 6A2 were involved in the utilization of alkanes with chain lengths ranging from C10 to C18 implying that other enzyme(s) should be required for the utilization of C20‐C40 alkanes.
In addition to the notorious cytochrome P450 monooxygenase for alkane hydroxylation, a group of novel extracellular heme‐thiolate peroxygenases secreted by fungal Agrocybe aegerita have been demonstrated to catalyze efficiently the H 2O 2‐dependent hydroxylation of a variety of alkanes, which include linear, branched, and cyclic saturated hydrocarbons. As shown in Scheme 2.9, linear C3‐C16 alkanes can be hydroxylated at either 2‐ or 3‐positon of the carbon chain to yield corresponding secondary alcohols. For example, n ‐propane and n ‐butane were hydroxylated regioselectively to produce 2‐propanol and 2‐butanol, whereas n ‐heptane and n ‐octane were hydroxylated both stereoselectively and regioselectively to give 99.9% e.e. ( R )‐enantiomer and the corresponding 3‐alcohol. Branched alkanes such as 2,3‐dimethylbutane were hydroxylated regioselectively to 2,3‐dimethylbutane‐2‐ol and isobutene was oxidized to 2‐methyl‐propan‐2‐ol. While cyclic alkanes of C5–C8 gave monohydroxylated products [48], there are also non‐heme enzymes that are capable of catalyzing the hydroxylation of alkanes [49]. Studies showed that non‐heme alkane hydroxylase involves high‐valent oxoiron moiety to catalyze the initial H abstraction from alkane followed by the rebound mechanism of FeOH/radical species. Examples are the enzyme taurine:α‐ketoglutarase dioxygenase (TauD) toward the cyclohexanol and cyclopentanol from cyclohexane and cyclopentane, respectively [50], and the diiron alkane monooxygenase of Pseudomonas oleovorans (AlkB) hydroxylates 2‐methyl‐1‐phenylcyclopropane to produce only 1‐phenyl‐3‐buten‐1‐ol and norcarane to produce approximately 85% cis ‐ and trans ‐2‐norcaranol [51].
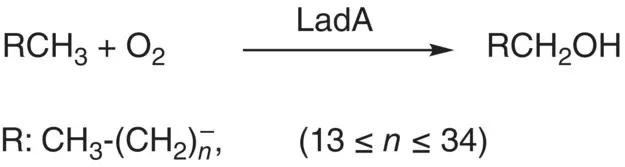
Scheme 2.8 Terminal hydroxylation of long‐chain alkanes by LadA.
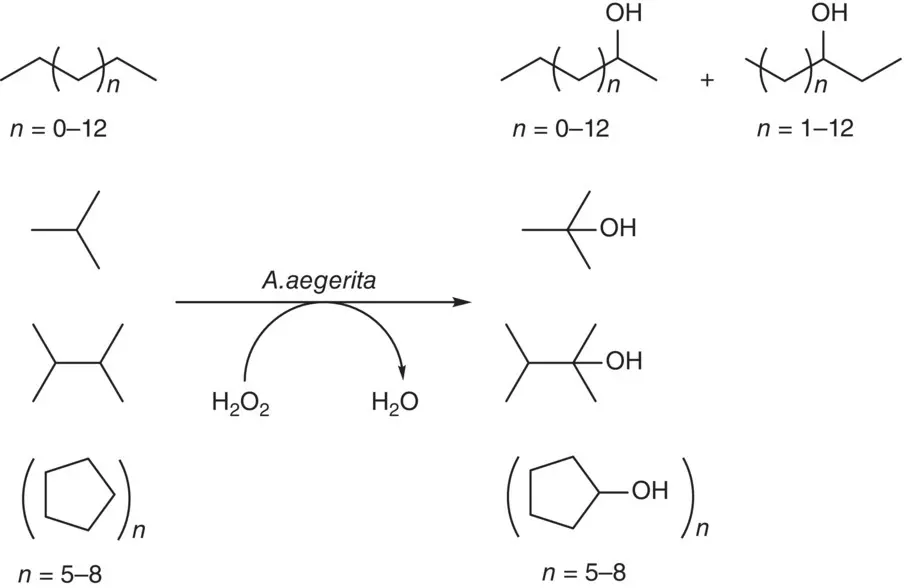
Scheme 2.9 Hydroxylation of alkanes by fungal peroxygenase.
2.1.3 Hydroxylation of Aromatic Compounds
Oxidative enzymes performing the introduction of one or two oxygen atoms on aromatic compounds is important in industrial applications [52, 53]. The four classes of enzyme: oxygenases, hydroxylases, peroxidases, and laccases are the most representative enzymes to perform the hydroxylation of aromatic compounds. The four classes of oxidative enzymes differ in many aspects, such as the metal present in the active site, the number of electrons transferred for the reaction, and the kind of reductive cofactor [54]. The most common hydroxylases are the cytochrome P‐450‐dependent hydroxylases and the flavoprotein phenol hydroxylases [55]. Since hydroxylations introduce the hydroxyl group into organic compounds primarily via the substitution of functional groups or hydrogen atoms, it is a great challenge to organic chemists to perform the direct and selective introduction of the hydroxyl group into aromatic ring.
One of the well‐known industrial applications with hydroxylase for the hydroxylation of aromatic ring is the catalytic production of tyrosine from phenylalanine by phenylalanine hydroxylase and the subsequent hydroxylation of tyrosine yielding the catecholamine neurotransmitter L‐dihydroxyphenylalanine (L‐DOPA), as in Scheme 2.10[53, 55].
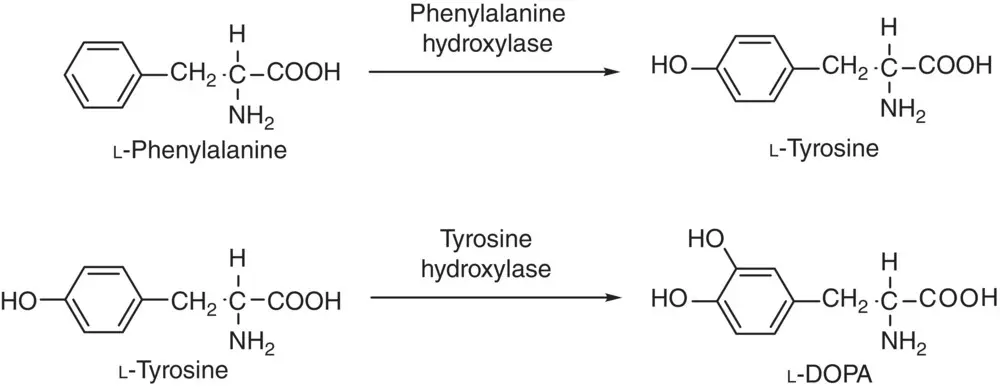
Scheme 2.10 The catalytic hydroxylation of L‐phenylalanine and L-tyrosine by phenyl hydroxylase and tyrosine hydroxylase, respectively, to produce corresponding L‐tyrosine and L‐DOPA.
Recently, a microsomal P450 designated as PcCYP65a2 that was derived from the white rot fungus Phanerochaete chrysosporium can catalyze 3′‐hydroxylation of naringenin to yield eriodictyol in the culture of the recombinant S. cerevisiae AH22/pG65a2 cells ( Scheme 2.11) [56].
Isoliquiritigenin (2′,4′,4‐trihydroxychalcone) is a chalcone found in licorice root and other plants, which has shown potent antioxidant, anti‐inflammatory, phytoestrogenic, tyrosinase inhibitory, and antitumor activity in vitro . However, when prepared in vivo , aromatic hydroxylation of isoliquiritigenin on the A or B ring was found by the metabolism of human liver microsomes to produce 2′,4′4′,5′‐tetrahydroxychalcone or butein, respectively ( Scheme 2.12) [57]. The enzyme involved in the formation of the hydroxylated metabolites of isoliquiritigenin was cytochrome P450.
Amphetamine was once extensively used for weight reduction, and it has been employed in treating mild depression and narcolepsy as well. The administration of amphetamine may cause excitability, restlessness, tremors, insomnia, dilated pupils, increased pulse rate and blood pressure, hallucinations, and psychoses. Since amphetamine is inexpensive, it has been a drug of abuse. The biotransformation of d ‐amphetamine into p ‐hydroxyamphetamine (HA) by cytochrome P450 occurs in human and mouse ( Scheme 2.13) [58]. The urinary excretion of HA not only is a biomarker of exposure but also gives an insight into d ‐amphetamine reactivity.

Scheme 2.11 Hydroxylation of naringenin in the culture of the recombinant S. cerevisiae expressing P. chrysosporium PcCYP65a2 to produce eriodictyol.
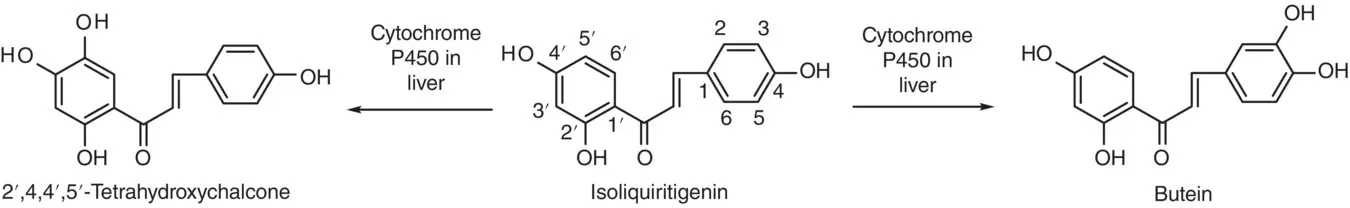
Scheme 2.12 Hydroxylation of isoliquiritigenin in human liver by cytochrome P450.
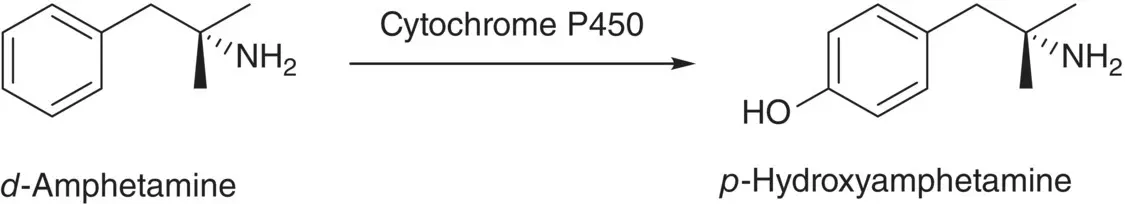
Scheme 2.13 Hydroxylation of d ‐amphetamine by cytochrome P450 to give p ‐hydroxyamphetamine.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Enzyme-Based Organic Synthesis»
Представляем Вашему вниманию похожие книги на «Enzyme-Based Organic Synthesis» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Enzyme-Based Organic Synthesis» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












