Cheanyeh Cheng - Enzyme-Based Organic Synthesis
Здесь есть возможность читать онлайн «Cheanyeh Cheng - Enzyme-Based Organic Synthesis» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Enzyme-Based Organic Synthesis
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Enzyme-Based Organic Synthesis: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Enzyme-Based Organic Synthesis»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
An insightful exploration of an increasingly popular technique in organic chemistry Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis
Enzyme-Based Organic Synthesis — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Enzyme-Based Organic Synthesis», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Direct oxidation of heterocyclic and aromatic aldehydes to the corresponding carboxylic acids can be accomplished by Acetobacter rancens IFO3297, Acetobacter pasteurianus IFO13753, and Serratia liquefaciens LF14 [1, 23]. For instance, oxidation of furfural by A. rancens IFO3297 can produce 110 g L −1of 2‐furoic acid with a 95% yield. 5‐Hydroxymethyl‐2‐furancarboxylic acid obtained from corresponding aldehyde can be obtained by whole cells LF14. Isophthalaldehyde, 2,5‐furandicarbaldehyde, 2,5‐thiophenedicarbaldehyde, and 2,2′‐biphenyldicarbaldehyde can be converted to the corresponding formylcarboxylic acid with 86–91% yields by both IFO13753 and LF14. The aromatic carboxylic acids such as vanillic acid, p ‐hydroxybenzoic acid, and syringic acid can be produced by the oxidation of corresponding aromatic aldehydes using whole‐cell Burkholderia cepacia TM1 [1, 24].
The oxidation of alcohols and aldehydes to form corresponding aldehydes, ketones, and carboxylic acids has also been used by cells to produce important intermediates or precursors during the biosynthetic pathways. In the biosynthesis of depside atranorin from acetate using immobilized lichen cells Evernia prunastri , the most probable precursor, an aldehyde‐substituted phenolic aldehyde, haematommic acid, is produced by oxidation of an alcohol intermediate as shown in Scheme 2.6[25]. In another example, resting cells of Nocardia iowensis DSM 45197 have been selected for the synthesis of vanillin and vanillic acid from the starting material isoeugenol. Three possible pathways used by N. iowensis have been suggested for the conversion of isoeugenol to vanillic acid and vanillin. 18O‐labeling studies showed that most likely route appears to be the initial side‐chain olefin epoxidation of isoeugenol, epoxide hydrolysis to a vicinal diol, followed by diol cleavage to vanillin and subsequently oxidation of the aldehyde group of vanillin to vanillic acid by an aldehyde oxidase ( Scheme 2.7) [26].
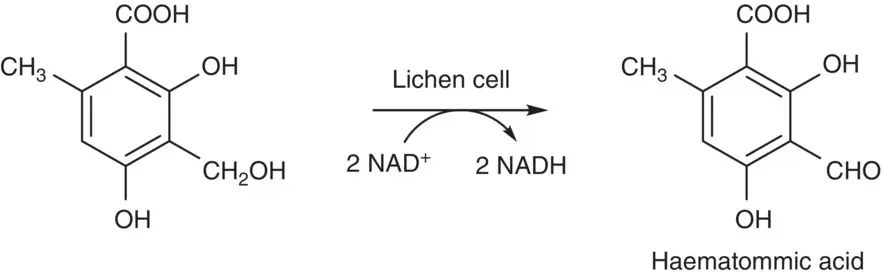
Scheme 2.6 Oxidation of an alcohol intermediate to the precursor of atranorin in the biosynthetic pathway of lichen cells.
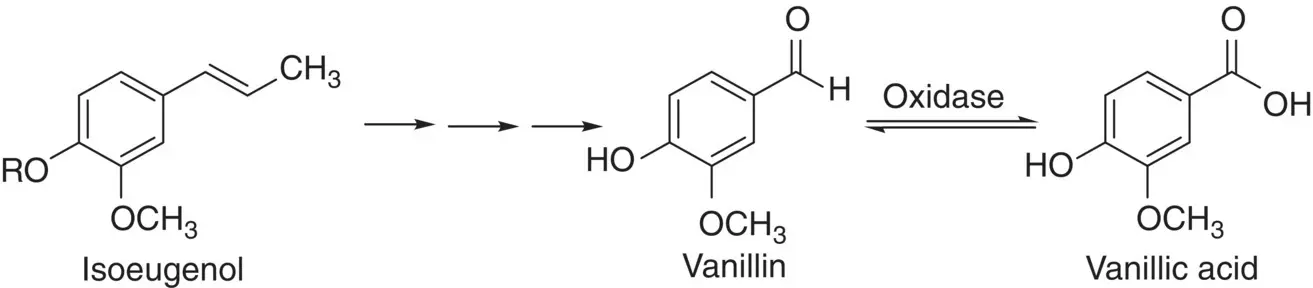
Scheme 2.7 Bioconversion of isoeugenol to vanillin and vanillic acid by N. iowensis .
2.1.2 Hydroxylation of Alkanes
Alkanes are saturated hydrocarbons that constitute about 20–50% of crude oil, and living organisms, such as bacteria, plants, and some animals, also produce them. They are chemically quite inert, low value, and usually burned as energy source to produce carbon oxides. Thus, there are two main reasons to carry out the catalytic hydroxylation of inert C–H bonds in alkanes for chemical industry applications. The first one is the providing of high‐value compounds from the low‐value oil refinery products such as the manufacturing of solvents, plasticizers, and surfactants. The second one is the removal of pollutants from the environment [27, 28].
Because carbon and hydrogen atoms have almost equal electronegativity, the activation of alkanes by the hydroxylation process, especially at the terminal positions, remains a challenging topic in synthetic chemistry. Despite the use of high‐temperature heterogeneous catalysts or environmentally unfriendly organometallic catalysis for the selective hydroxylation of alkanes, biocatalysts provide an alternative approach that has the advantages of high selectivity, mild reaction conditions, and low by‐product formation [29]. Nature has demonstrated the ability of biocatalysts for the hydroxylation of alkanes that many microorganisms have evolved enzymes for activating alkanes by oxidation of one of the terminal methyl groups to generate the corresponding primary alcohol in the presence of oxygen. The formation of primary alcohol with oxidative enzymes can be further oxidized to fatty acid by dehydrogenases and further metabolized. Oxidative enzymes are called oxygenases that can catalyze the selective insertion of oxygen atoms into a wide range of organic compounds. Oxygenases such as methane monooxygenase, alkane hydroxylase, and cytochrome P450 are the common biocatalysts used for the oxidation of a variety of alkanes [30].
The diversity of alkane oxygenases evolved in prokaryotes and eukaryotes has been strategically used in synthetic chemistry to catalyze the chemo‐, regio‐, and stereoselective oxygenation of alkanes and many other compounds for the production of useful alcohols, aldehydes, epoxides, and carboxylic acids [31]. In particular, cytochromes P450 are external monooxygenases that convert a broad variety of substrates and catalyze many interesting chemical reactions including the hydroxylation of hydrocarbons [32, 33]. As an example, cytochrome P450 BM3(CYP102A1) isolated from Bacillus megaterium catalyzes the hydroxylation of gaseous alkanes, such as methane, ethane, propane, and butane, and cyclohexane to corresponding primary alcohols and 2‐propanol and 2‐butanol together with a dummy substrate perfluorocarboxylic acids of different alkyl chain length (8–14 carbon atoms) [34]. The screening of the mutant library revealed that cytochrome P450 BM3 F87a/A328V mutant has the ability to effectively hydroxylate cyclooctane, cyclodecane, and cyclododecane to corresponding alcohols. Cytochrome P450 BM3 F87V/A328F mutant demonstrated the ability to hydroxylate acylic n ‐octane to 2‐( R )‐octane with 46% e.e. and high regioselectivity of 92% [35]. For cytochrome P450 catalysis, the nicotinamide adenine dinucleotide cofactor in its reduced form (NADH or NADPH) is needed to provide the electrons necessary for the catalytic cycle. However, the transfer of electrons cannot be made directly and a cytochrome P450 reductase must be present to shuttle the electrons from NAD(P)H to the hydrolase domain ( Figure 2.1). It was discovered that cytochrome P450 BM3 was the first natural self‐sufficient P450, which still makes it one of the most efficient cytochromes to date [36].
Medium‐chain alkanes (C 5–C 16) are usually oxidized by heme‐iron‐containing cytochrome P450 monooxygenases (P450s or CYPs) or by integral‐membrane non‐heme diiron monooxygenases (Alk B) [37, 38]. Alkane hydrolases of the CYP153A from Mycobacterium marinum (CYP153A16) and Polaromona sp. (CYP153A P . sp.) were examples to catalyze regioselectively ω‐hydrolation of C 6–C 11alkanes, alkenes, cycloalkanes, and alicyclic compounds [39, 40]. The cloned and expressed CYP153A16 and CYP153A P . sp. monooxygenases were capable of performing the ω‐hydroxylation in vitro with C 5–C 12alkanes and C 6–C 12primary alcohols into their corresponding primary alcohols and α,ω‐diols [37]. However, a soluble three‐component diiron monooxygenase, butane monooxygenase (sBMO), has been purified from the gram‐negative β‐proteobacterium Pseudomonas butanovora (ATCC 43655), which was capable of hydroxylating C 3–C 6linear and branched aliphatic alkanes (propane, butane, pentane, hexane, isobutene, and isopentane) at the terminal carbon atom mostly (≥80% regiospecificity) to produce primary alcohols with only small fractions of secondary and tertiary alcohols [41].
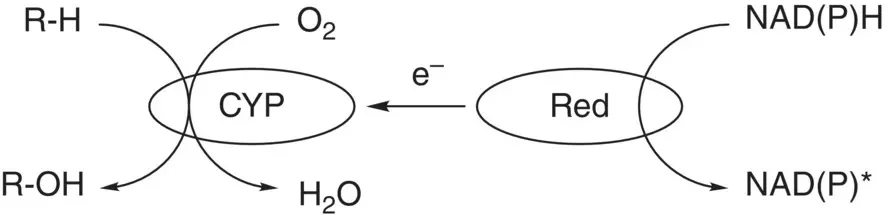
Figure 2.1 Hydroxylation of alkanes by cytochrome P450 monooxygenase (CYP).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Enzyme-Based Organic Synthesis»
Представляем Вашему вниманию похожие книги на «Enzyme-Based Organic Synthesis» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Enzyme-Based Organic Synthesis» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












